Abstract
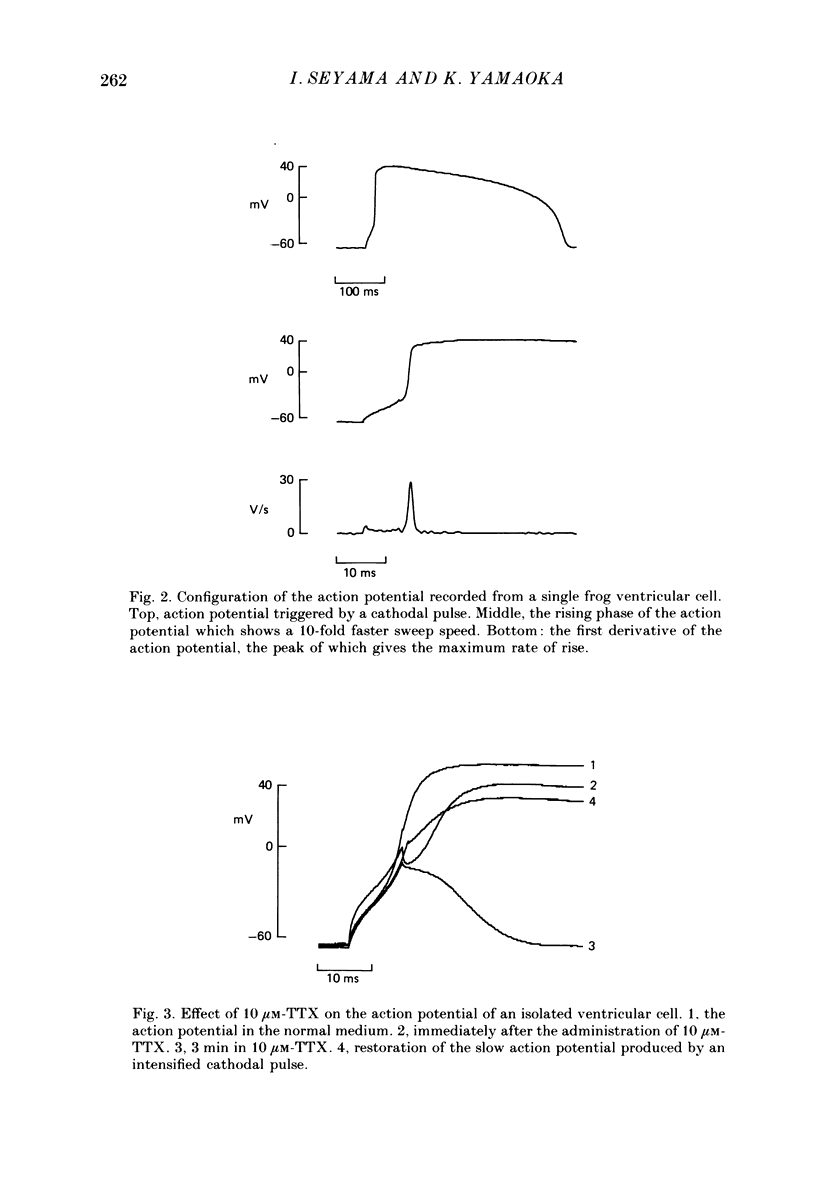
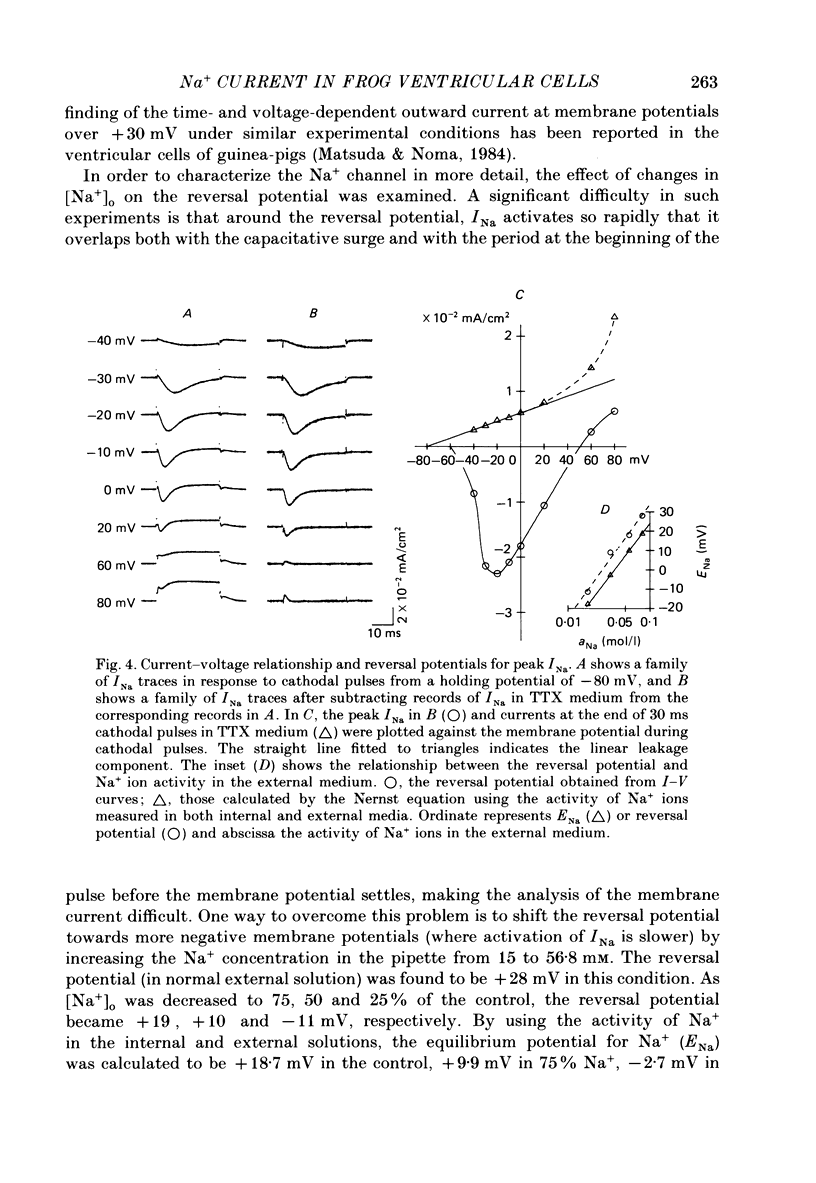
1. The generation of action potentials elicited from enzymatically dispersed ventricular cells from the frog, Rana catesbeiana, has been shown to be due to the influx of both Na+ and Ca2+. The maximum rate of rise, the amplitude and the duration at 50% repolarization of the action potential were estimated to be 26.4 +/- 5.1 V/s (n = 8), 110 +/- 2.7 mV (n = 8) and 601 +/- 180 ms (n = 8) at 15 degrees C, respectively. 2. Inward Na+ current (INa) was studied in these ventricular cells by the whole-cell patch clamp technique in a medium where Ca2+ current was eliminated by substituting extracellular Mg2+ for Ca2+ and K+ current was suppressed by applying Cs+ intracellularly. All the voltage clamp experiments were carried out at 4 degrees C. 3. INa elicited by single depolarizing steps from a holding potential (VH) of -80 mV had a threshold of -50 mV and was maximal at -20 mV. Peak currents in normal Ringer solution containing 113.5 mM-Na+ were of the order of 0.01-0.02 mA/cm2. Maximum Na+ conductance (gNa) was calculated to be 5.9 mS/cm2. 4. Under normal conditions the reversal potential for INa was determined to be 50 mV, which is close to the value predicted from the Nernst equation. The reversal potential changed by 59 mV per tenfold change in the activity of extracellular Na+ (aNa). 5. The instantaneous relation between INa tail currents and membrane potential is linear, crossing the abscissa at the reversal potential for INa. 6. Reconstructions of INa were made in terms of the parameters of the Hodgkin-Huxley model for the squid axon, using constants obtained from the frog ventricular cells. 7. The falling phase of INa and the development of inactivation measured by the double-pulse method could be well fitted by a single-exponential function. 8. The time course for recovery of INa from inactivation exhibited a single time constant.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Senft J. P. Dynamic asymmetries in the squid axon membrane. J Gen Physiol. 1968 May 1;51(5):102–114. [PMC free article] [PubMed] [Google Scholar]
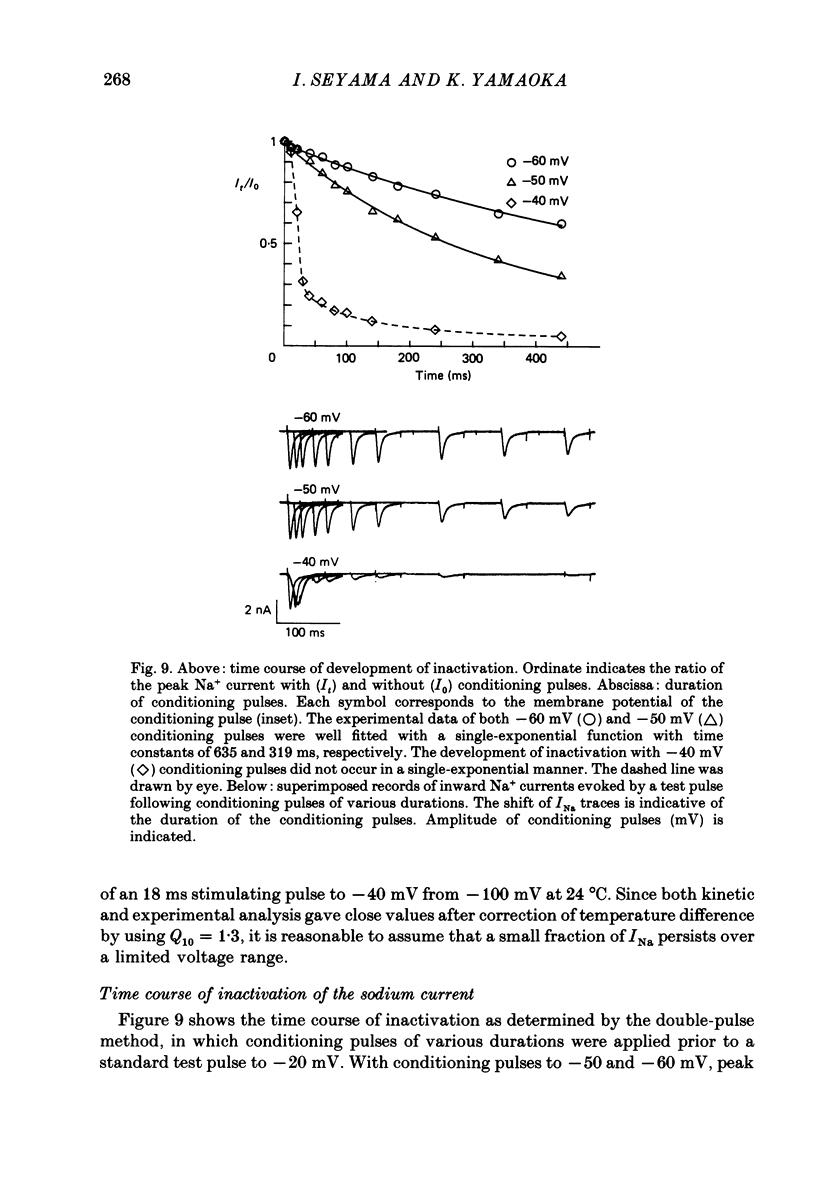
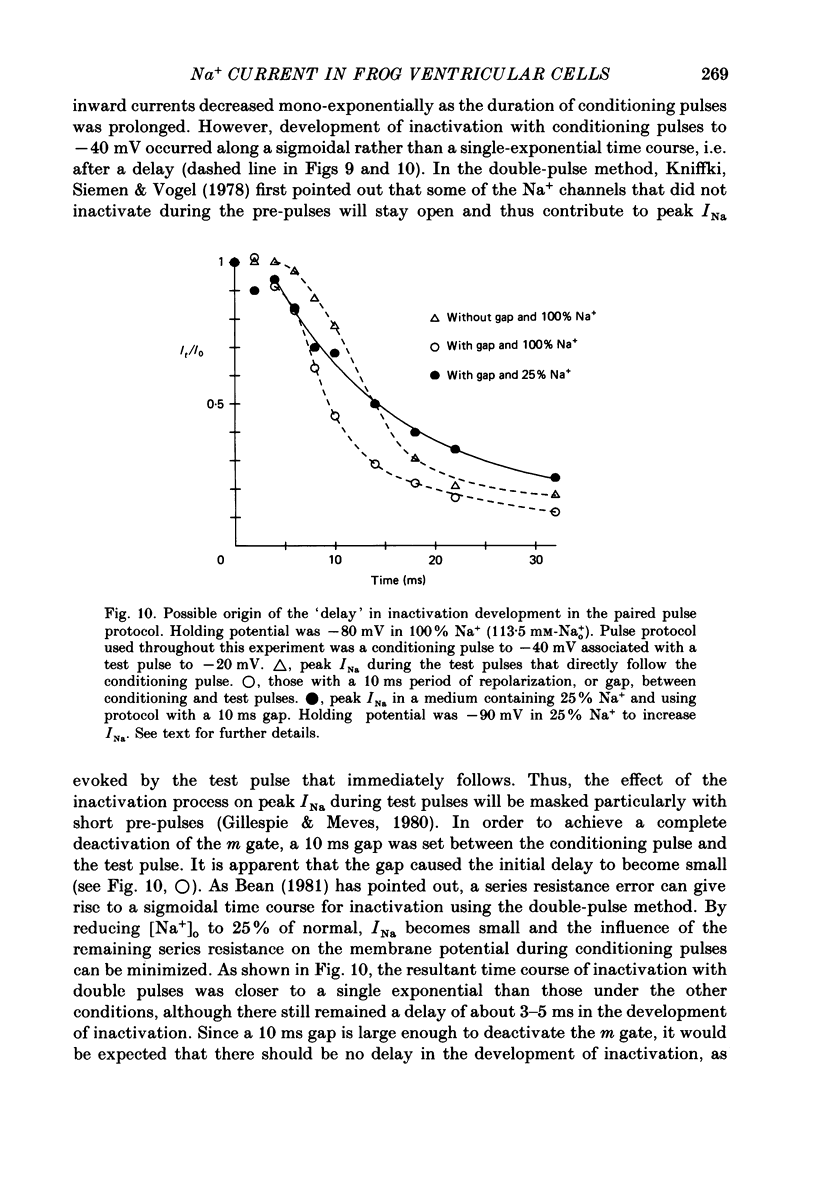
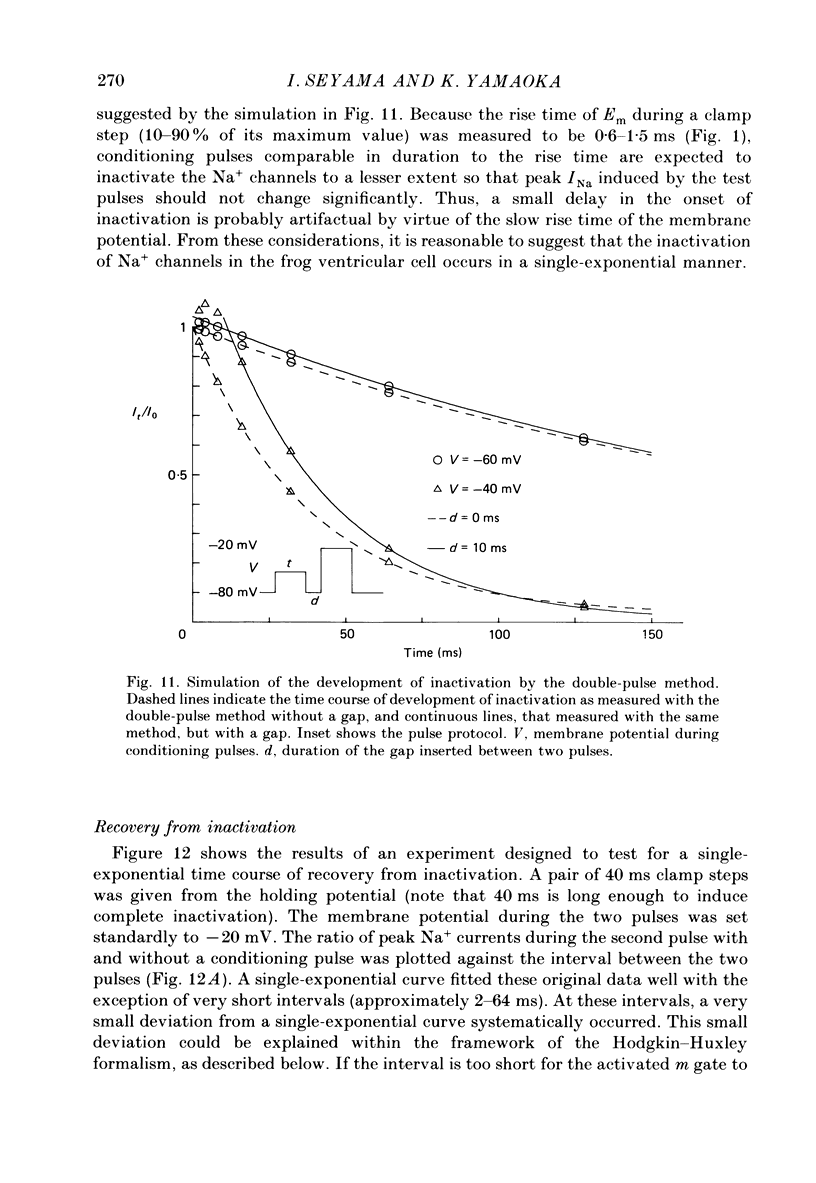
- Bean B. P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys J. 1981 Sep;35(3):595–614. doi: 10.1016/S0006-3495(81)84815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
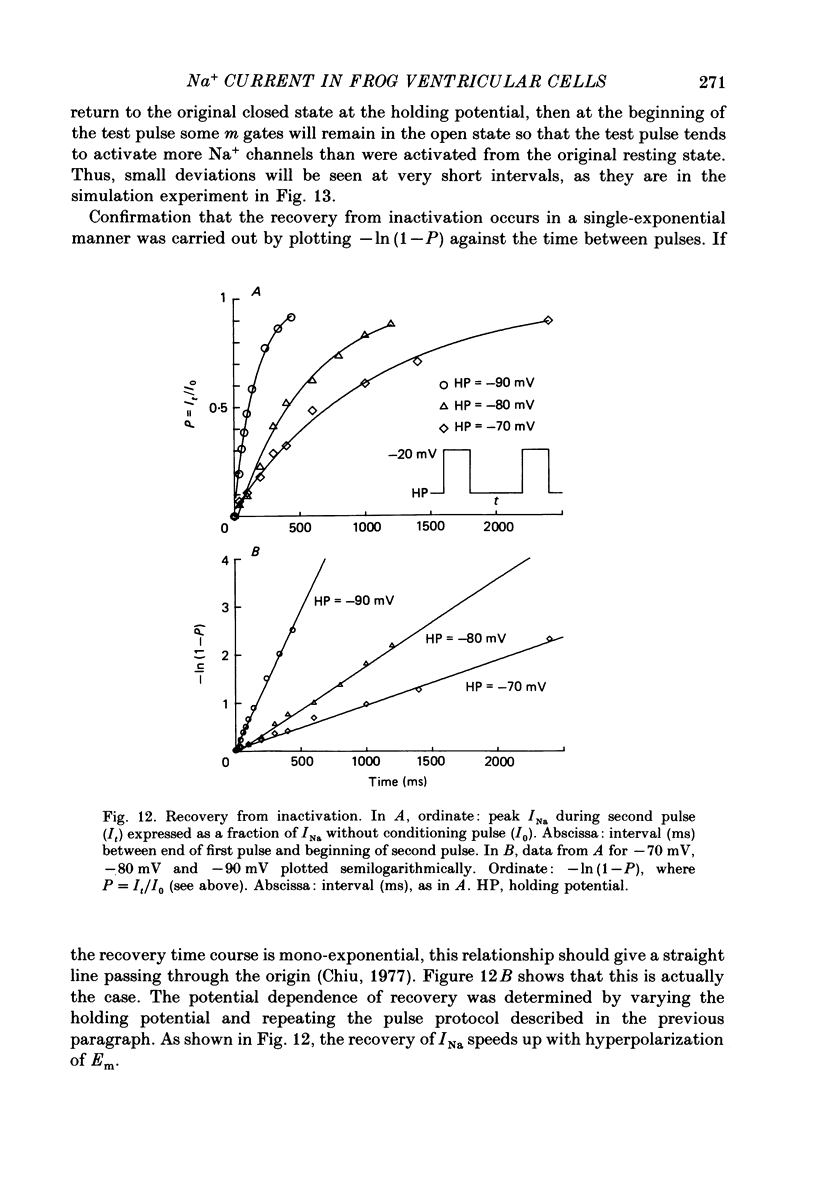
- Benndorf K., Boldt W., Nilius B. Sodium current in single myocardial mouse cells. Pflugers Arch. 1985 May;404(2):190–196. doi: 10.1007/BF00585418. [DOI] [PubMed] [Google Scholar]
- Bodewei R., Hering S., Lemke B., Rosenshtraukh L. V., Undrovinas A. I., Wollenberger A. Characterization of the fast sodium current in isolated rat myocardial cells: simulation of the clamped membrane potential. J Physiol. 1982 Apr;325:301–315. doi: 10.1113/jphysiol.1982.sp014151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A. J., Woodbury J. W. The sodium-potassium hypothesis as the basis of electrical activity in frog ventricle. J Physiol. 1960 Dec;154(2):385–407. doi: 10.1113/jphysiol.1960.sp006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Voltage clamp and internal perfusion of single rat heart muscle cells. J Physiol. 1981 Sep;318:455–477. doi: 10.1113/jphysiol.1981.sp013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J. O., McDonald T. F. Sodium currents in segments of human heart cells. Science. 1983 Apr 15;220(4594):320–321. doi: 10.1126/science.6301004. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Carmeliet E., Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. J Physiol. 1984 Apr;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky T. J. Voltage clamp measurements of sodium channel properties in rabbit cardiac Purkinje fibres. J Physiol. 1980 Aug;305:215–234. doi: 10.1113/jphysiol.1980.sp013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. G., Kern R., Einwächter H. M., Tarr M. Kinetics of Na inactivation in frog atria. Pflugers Arch. 1971;323(2):141–157. doi: 10.1007/BF00586445. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972 Jun;59(6):637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983 Feb;81(2):153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Isolated bovine ventricular myocytes. Characterization of the action potential. Pflugers Arch. 1982 Oct;395(1):19–29. doi: 10.1007/BF00584964. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Calcium channels in the neuronal membrane. Biochim Biophys Acta. 1981 Dec;650(2-3):128–150. doi: 10.1016/0304-4157(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Weeks T. A., Kao R. L., Akaike N., Brown A. M. Sodium current in single heart muscle cells. Nature. 1979 Mar 15;278(5701):269–271. doi: 10.1038/278269a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki N., Hermsmeyer K. Tetrodotoxin-sensitive Na+ channels in isolated single cultured rat myocardial cells. Am J Physiol. 1983 Nov;245(5 Pt 1):C381–C387. doi: 10.1152/ajpcell.1983.245.5.C381. [DOI] [PubMed] [Google Scholar]
- Page S. G., Niedergerke R. Structures of physiological interest in the frog heart ventricle. J Cell Sci. 1972 Jul;11(1):179–203. doi: 10.1242/jcs.11.1.179. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets M. F., January C. T., Fozzard H. A. Isolation and characterization of single canine cardiac purkinje cells. Circ Res. 1983 Oct;53(4):544–548. doi: 10.1161/01.res.53.4.544. [DOI] [PubMed] [Google Scholar]
- Staley N. A., Benson E. S. The ultrastructure of frog ventricular cardiac muscle and its relationship to mechanism of excitation-contraction coupling. J Cell Biol. 1968 Jul;38(1):99–114. doi: 10.1083/jcb.38.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Seyama I. Some properties of Na channel inactivation in isolated ventricular cells of frog, Rana catesbeiana. Jpn Heart J. 1986 Nov;27 (Suppl 1):21–30. [PubMed] [Google Scholar]


