Abstract
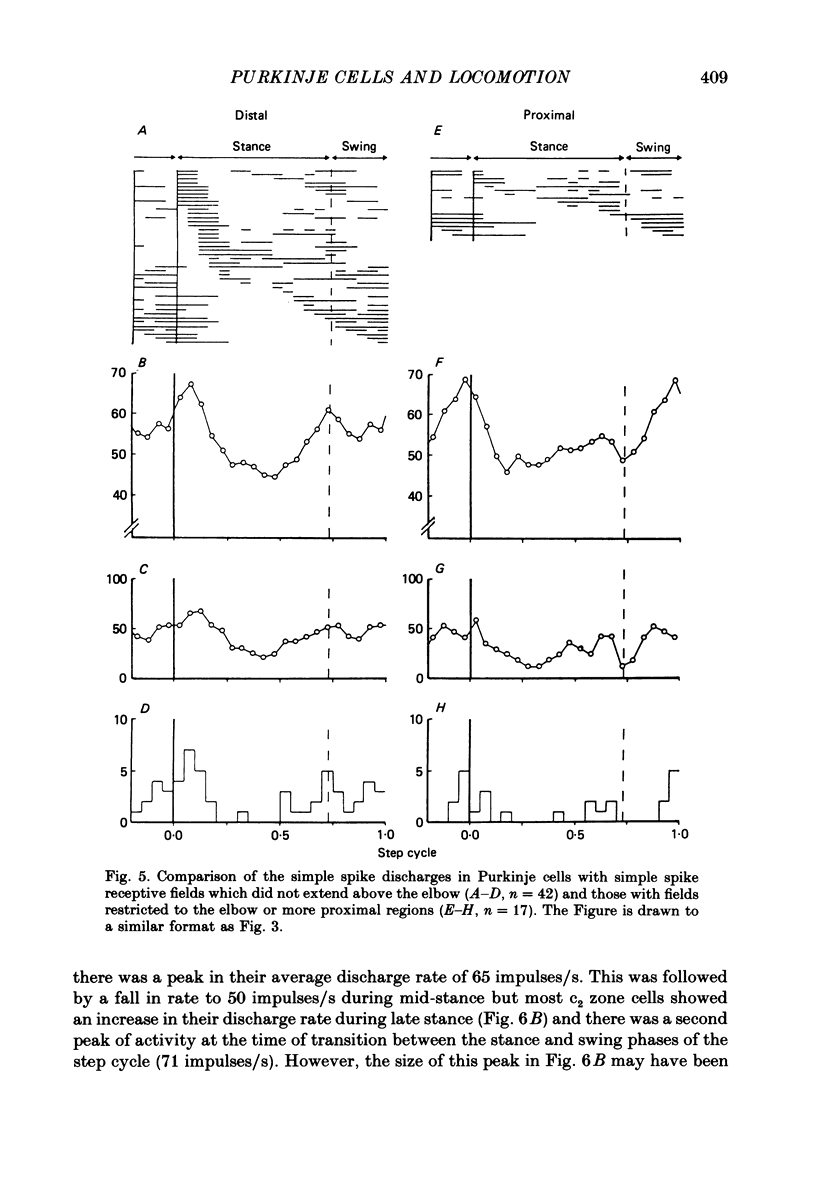
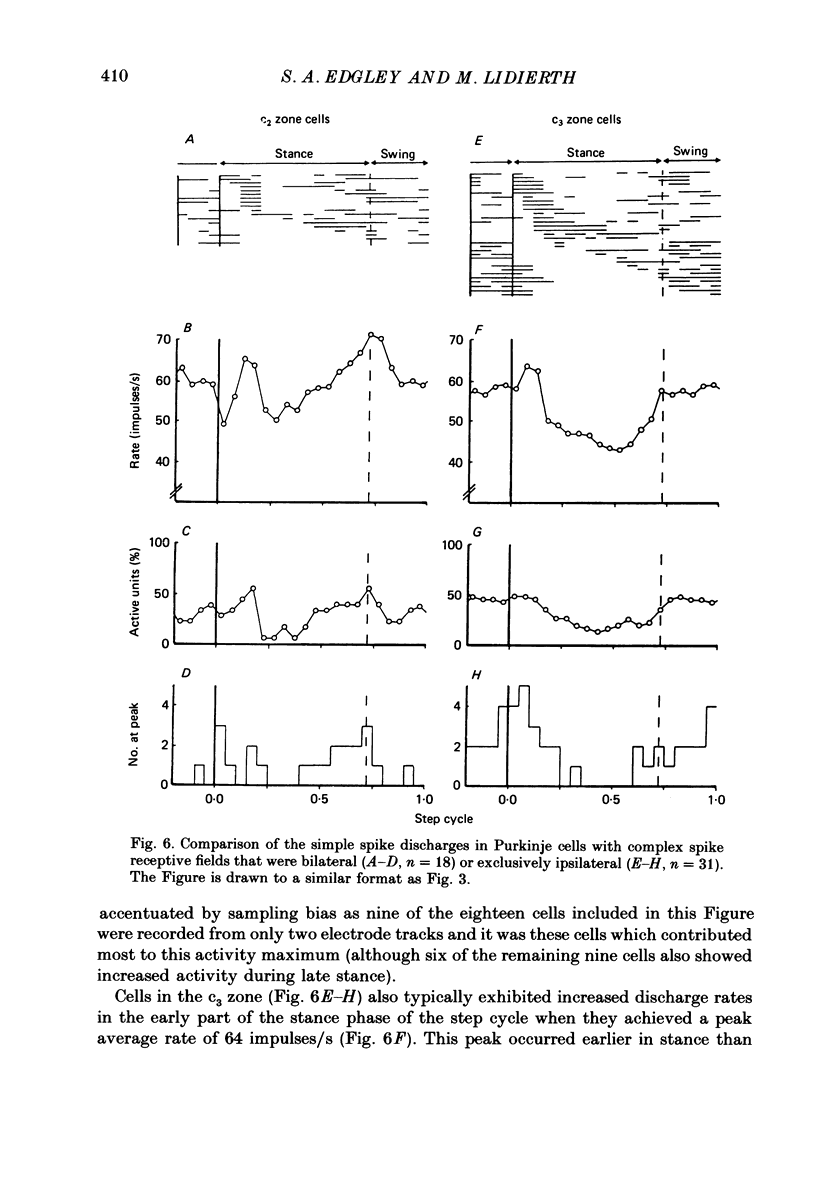
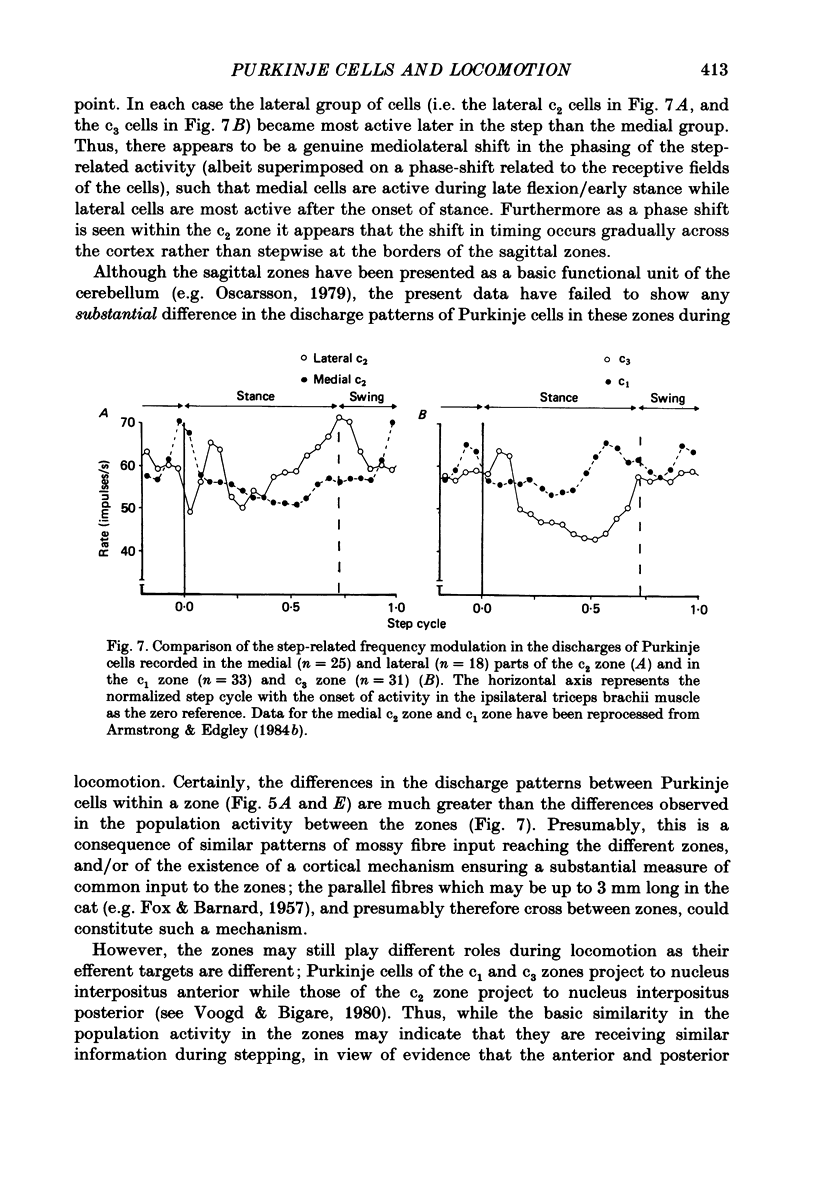
1. Extracellular recordings were made of the simple spike discharges of Purkinje cells in the lateral part of the paravermal cortex of lobule V in the cerebellum of awake cats. The cells were located within the c2 and c3 zones of Oscarsson (1979). 2. The peripheral receptive fields in which light mechanical stimuli could evoke simple spikes were examined in 252 Purkinje cells. Ninety-two per cent were activated by stimulation of the ipsilateral forelimb and 52% of 113 tested cells also discharged simple spikes in response to stimulation of the contralateral forelimb. The receptive fields were concentrated on the distal parts of the limbs: 67% of the 139 cells which were examined in most detail responded to stimulation of the paw or wrist of the ipsilateral forelimb. 3. In 135 of the Purkinje cells, the discharges were recorded during locomotion. Simple spikes were discharged at a mean rate of 54.3 +/- 27.8 impulses/s (S.D., n = 135) during steady walking on a belt moving at 0.5-0.7 m/s. The discharges of each cell were rhythmically modulated in time with the movements of stepping and although the timings of the discharges were highly variable between cells, activity in the population was greatest at the times of transition between the stance and swing phases in the ipsilateral forelimb and least during mid-stance. 4. As a population Purkinje cells with simple spike receptive fields on the distal parts of the forelimb(s) exhibited two activity maxima. These occurred during early stance and during the transition from stance to swing in the ipsilateral forelimb. Cells with receptive fields on the proximal parts of the limb achieved an activity maximum during late swing, and their average discharge rate fell at the time of onset of the swing phase in the ipsilateral forelimb instead of rising as was the case for the distal group. 5. The present results are compared with those from cells located more medially in the paravermal cortex. It is shown that medially located cells tend to discharge earlier in stance (or in late flexion) than laterally located cells with similar receptive fields.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol. 1984 Jul;352:403–424. doi: 10.1113/jphysiol.1984.sp015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol. 1984 Jun;351:411–432. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A., Lidierth M. Complex spikes in Purkinje cells of the paravermal part of the anterior lobe of the cat cerebellum during locomotion. J Physiol. 1988 Jun;400:405–414. doi: 10.1113/jphysiol.1988.sp017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Leonard P., Rawson J. A. A simple micromanipulator for investigation of cerebellar neurones in unrestrained cats. J Physiol. 1975 Feb;245(2):23P–25P. [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979 Apr;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal A., Kawamura K. Olivocerebellar projection: a review. Adv Anat Embryol Cell Biol. 1980;64:IVIII, 1-140. [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955 Aug;103(1):105–129. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955 Dec;74(6):653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- Campbell N. C., Armstrong D. M. Origin in the medial accessory olive of climbing fibres to the x and lateral c1 zones of the cat cerebellum: a combined electrophysiological/WGA-HRP investigation. Exp Brain Res. 1985;58(3):520–531. doi: 10.1007/BF00235868. [DOI] [PubMed] [Google Scholar]
- Cooke J. D., Larson B., Oscarsson O., Sjölund B. Origin and termination of cuneocerebellar tract. Exp Brain Res. 1971 Oct 25;13(4):339–358. doi: 10.1007/BF00234336. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Integration by Purkyne cells of mossy and climbing fiber inputs from cutaneous mechanoreceptors. Exp Brain Res. 1972 Oct 29;15(5):498–520. doi: 10.1007/BF00236405. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. Termination in overlapping sagittal zones in cerebellar anterior lobe of mossy and climbing fiber paths activated from dorsal funiculus. Exp Brain Res. 1980 Jan;38(2):163–172. doi: 10.1007/BF00236737. [DOI] [PubMed] [Google Scholar]
- FOX C. A., BARNARD J. W. A quantitative study of the Purkinje cell dendritic branchlets and their relationship to afferent fibres. J Anat. 1957 Jul;91(3):299–313. [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. The natural discharges of Purkinje cells in paravermal regions of lobules V and VI of the monkey's cerebellum. J Physiol. 1977 Oct;271(2):515–536. doi: 10.1113/jphysiol.1977.sp012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson J. A., McCarley R. W. Spontaneous discharge rates of cat cerebellar Purkinje cells in sleep and waking. Electroencephalogr Clin Neurophysiol. 1972 Nov;33(5):457–469. doi: 10.1016/0013-4694(72)90210-6. [DOI] [PubMed] [Google Scholar]
- Lidierth M. A computer based method for automated measurement of the periods of muscular activity from an EMG and its application to locomotor EMGs. Electroencephalogr Clin Neurophysiol. 1986 Oct;64(4):378–380. doi: 10.1016/0013-4694(86)90163-x. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Mizuno N., Itoh K. An autoradiographic study on the terminal distribution of cerebellothalamic fibers in the cat. Brain Res. 1981 Jun 29;215(1-2):29–47. doi: 10.1016/0006-8993(81)90489-3. [DOI] [PubMed] [Google Scholar]
- Thach W. T., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967 Jul;30(4):675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- Udo M., Kamei H., Matsukawa K., Tanaka K. Interlimb coordination in cat locomotion investigated with perturbation. II. Correlates in neuronal activity of Deiter's cells of decerebrate walking cats. Exp Brain Res. 1982;46(3):438–447. doi: 10.1007/BF00238638. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Minoda K., Oda Y. Simple and complex spike activities of Purkinje cells during locomotion in the cerebellar vermal zones of decerebrate cats. Exp Brain Res. 1981;41(3-4):292–300. doi: 10.1007/BF00238886. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Oda Y. Cerebellar control of locomotion: effects of cooling cerebellar intermediate cortex in high decerebrate and awake walking cats. J Neurophysiol. 1980 Jul;44(1):119–134. doi: 10.1152/jn.1980.44.1.119. [DOI] [PubMed] [Google Scholar]


