Abstract
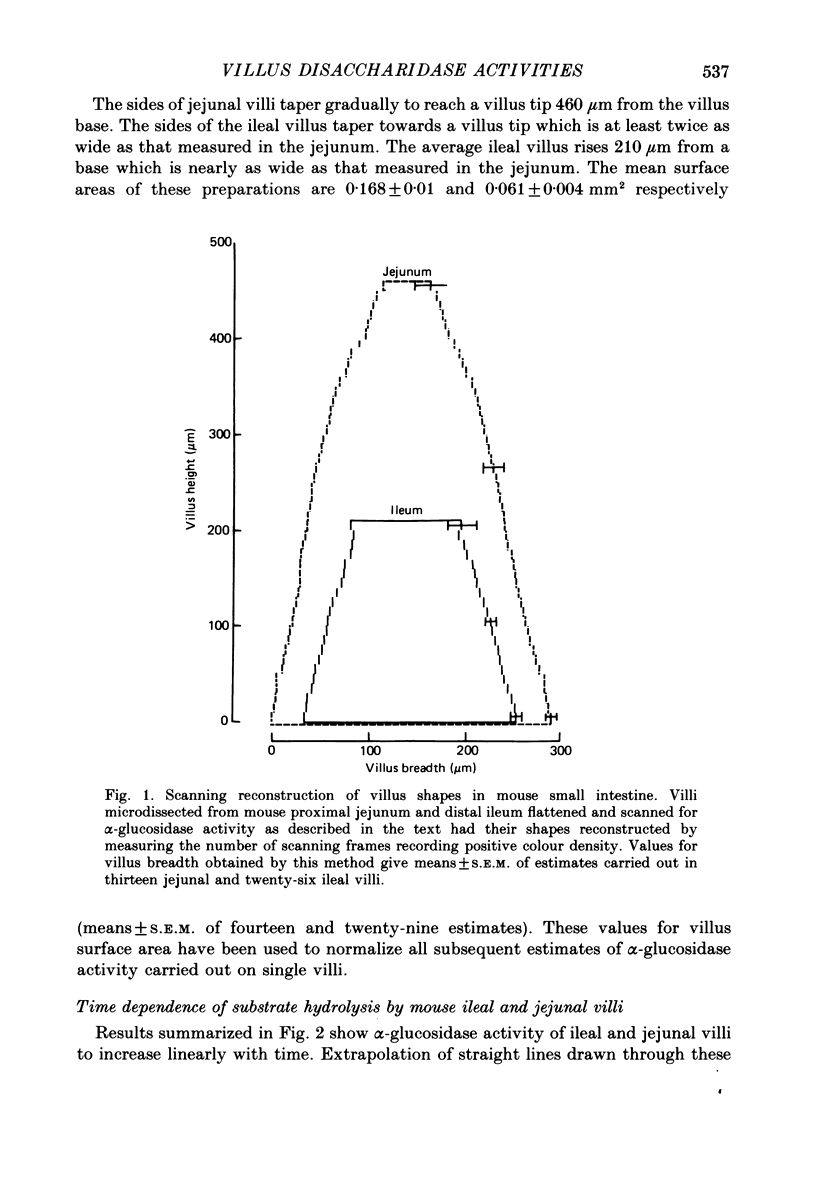
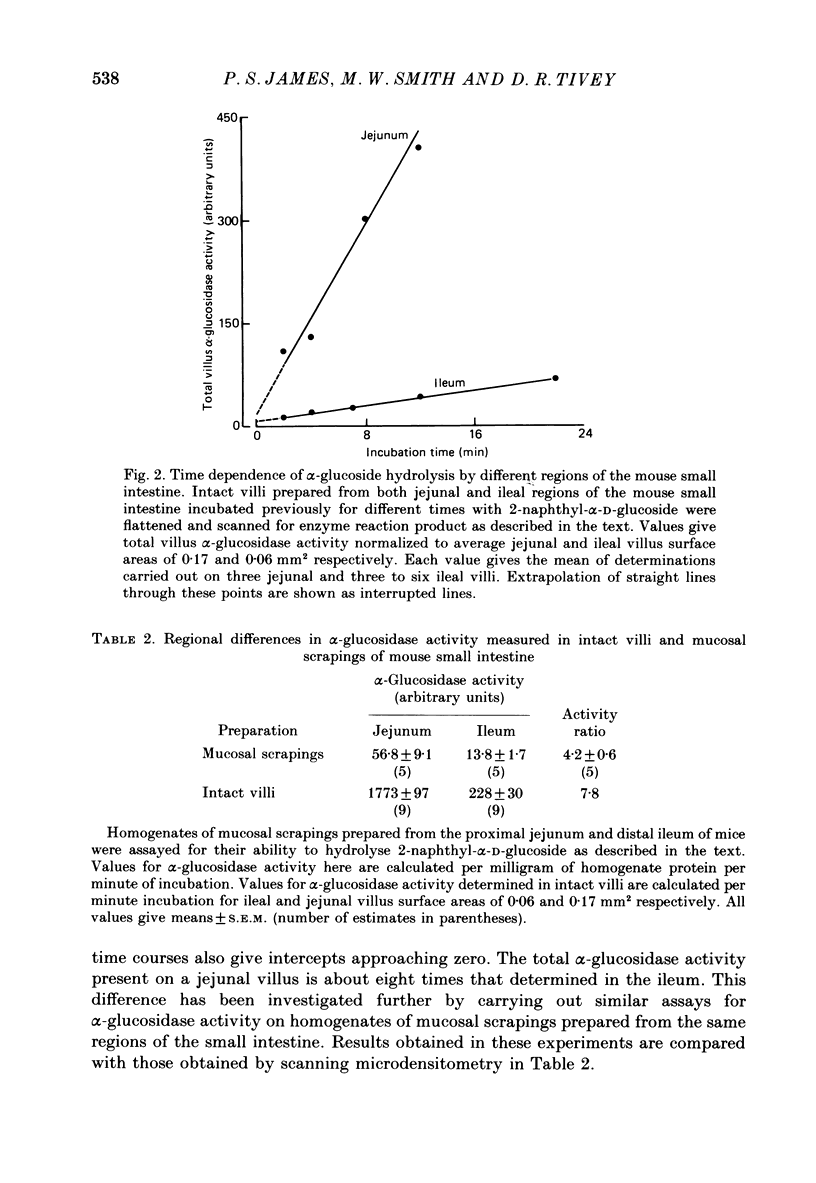
1. The present results describe how a new technique of whole-tissue cytochemistry can be combined with automatic scanning of microdissected villi to measure the capacity of individual villi to hydrolyse disaccharides in different parts of the small intestine. 2. Intact villi from the mouse proximal jejunum are found to be eight times more effective than ileal villi in hydrolysing 2-naphthyl-alpha-D-glucoside, an artificial substrate for enzymes normally hydrolysing sucrose, maltose, isomaltose and trehalose in adult intestine. Homogenates of jejunal scrapings are four times more effective than ileal homogenates in hydrolysing this substrate. This discrepancy arises from relating enzyme activities to homogenate protein in cases where intestinal structure changes. 3. The eightfold difference in villus alpha-glucosidase activity is associated with a threefold difference in villus surface area. This discrepancy in turn reflects changes in the capacity of individual enterocytes to express alpha-glucosidase during migration along the crypt-villus axis. These results emphasize the futility of trying to gauge intestinal function from measurement of intestinal structure. 4. Differences between ileal and jejunal villus alpha-glucosidase activities have been further partitioned into those depending on villus structure and those depending on enterocyte development. Present results are discussed in relation to the ability of luminal nutrition to maintain a proximal-distal gradient of digestive enzyme function in the small intestine. The general applicability of this method of analysis to other studies of adaptive response is also emphasized.
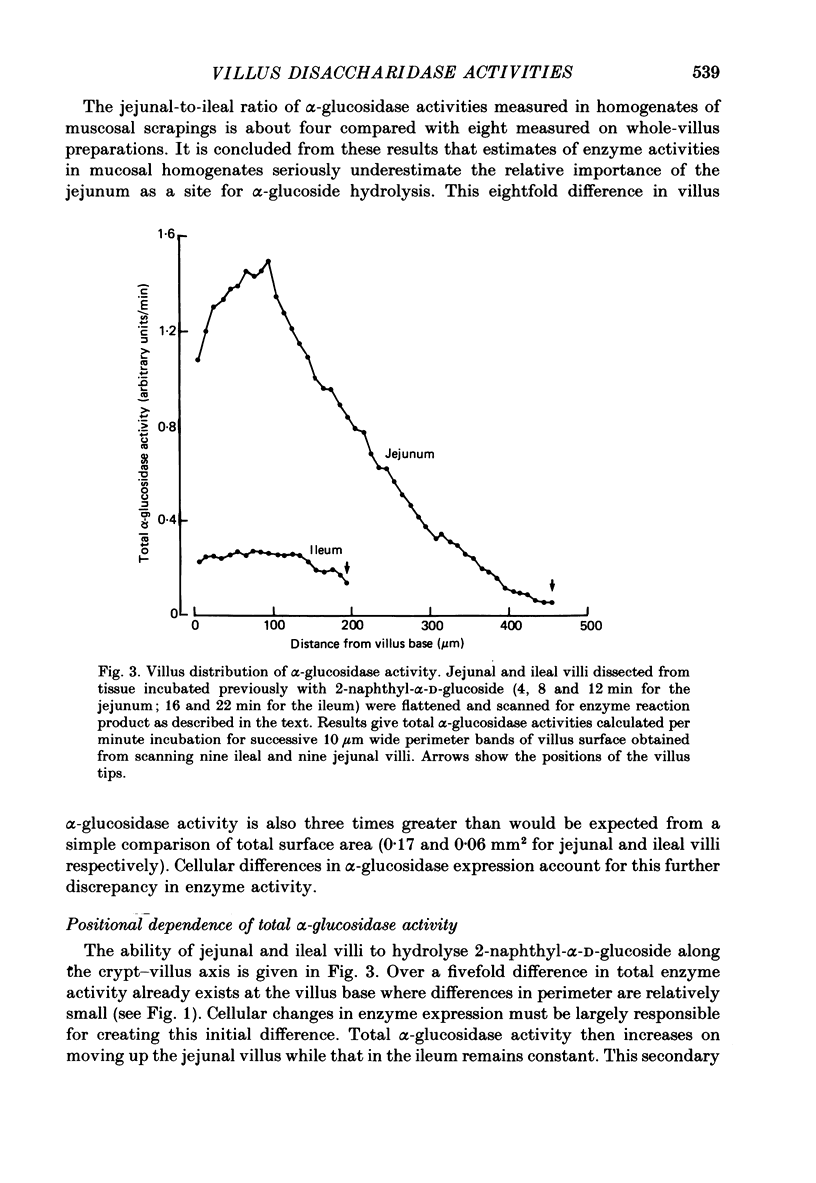
Full text
PDF



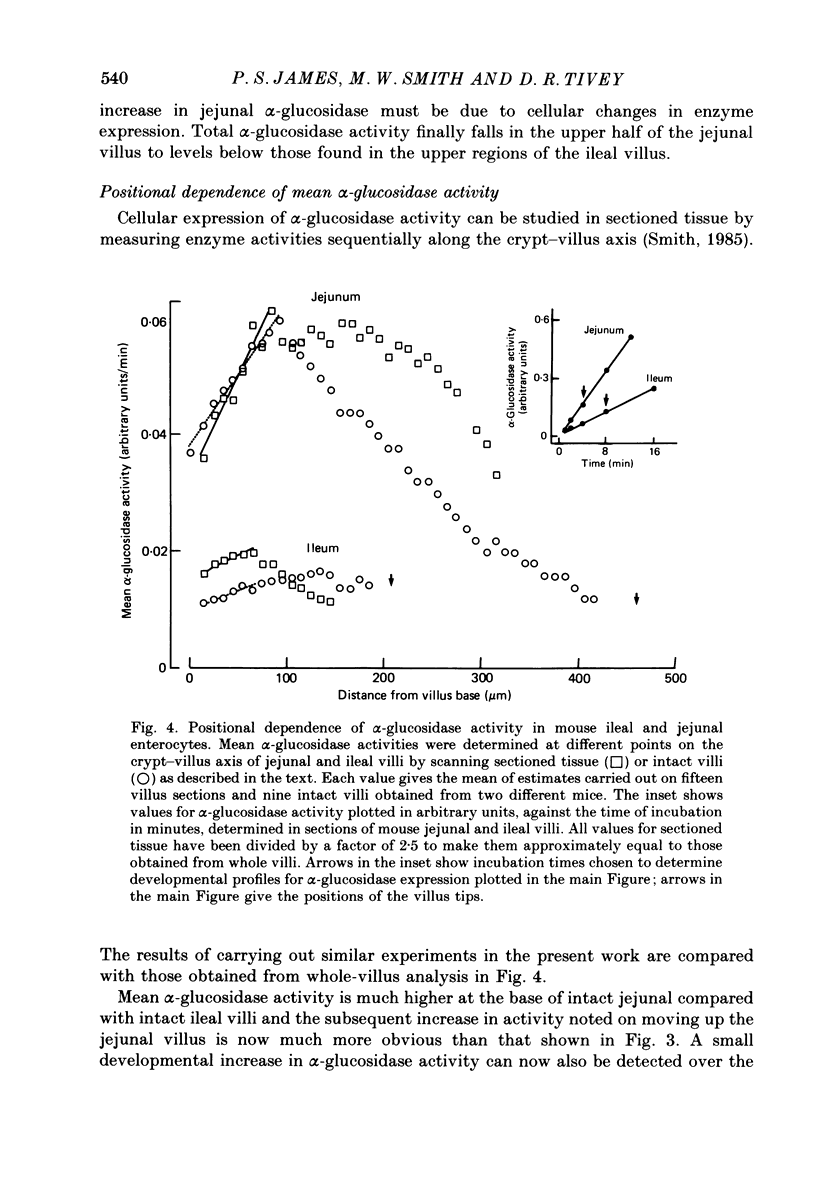

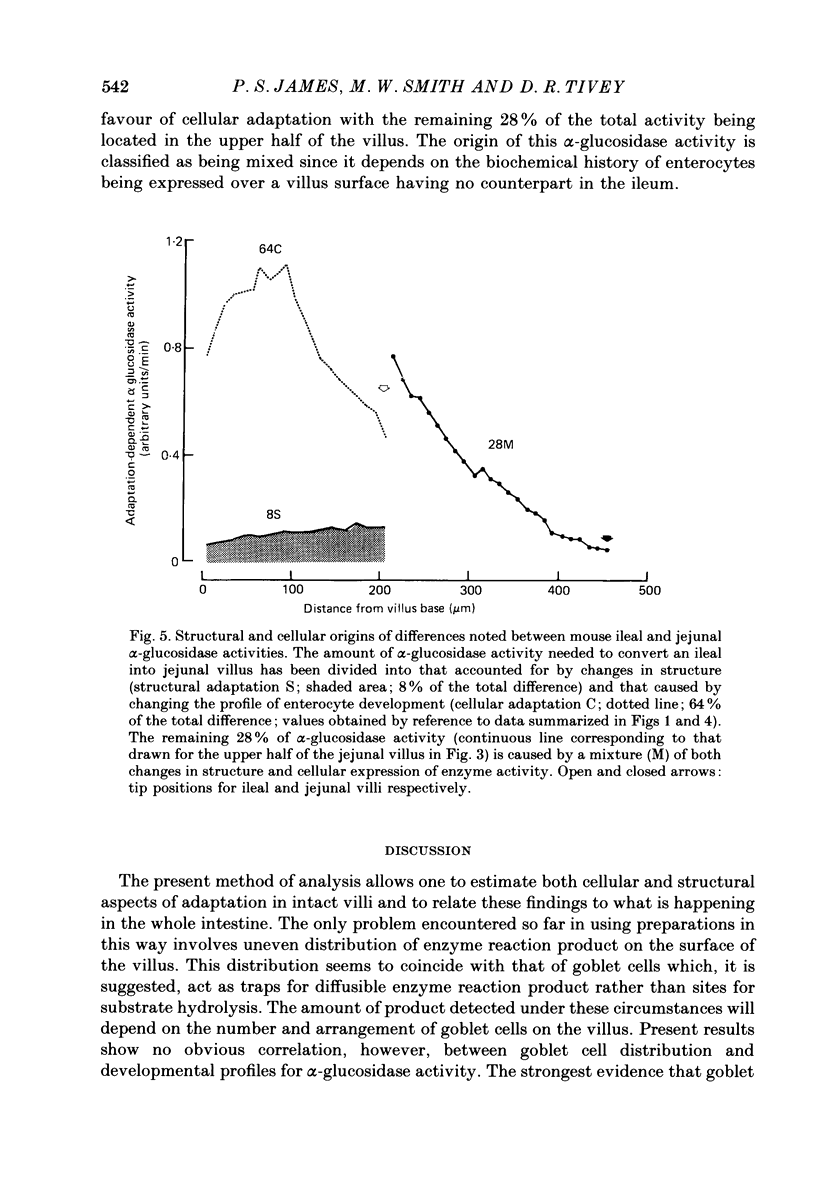



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G., Leblond C. P. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. Am J Anat. 1970 Jan;127(1):15–36. doi: 10.1002/aja.1001270104. [DOI] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. Mucous cells and cell migration in the mouse duodenal epithelium. Anat Rec. 1985 May;212(1):69–73. doi: 10.1002/ar.1092120110. [DOI] [PubMed] [Google Scholar]
- Bury K. D. Carbohydrate digestion and absorption after massive resection of the small intestine. Surg Gynecol Obstet. 1972 Aug;135(2):177–187. [PubMed] [Google Scholar]
- Chaves M., Smith M. W., Williamson R. C. Increased activity of digestive enzymes in ileal enterocytes adapting to proximal small bowel resection. Gut. 1987 Aug;28(8):981–987. doi: 10.1136/gut.28.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauncey M. J., Ingram D. L., James P. S., Smith M. W. Modification by diet and environmental temperature of enterocyte function in piglet intestine. J Physiol. 1983 Aug;341:441–452. doi: 10.1113/jphysiol.1983.sp014815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Dowling R. H. Small bowel adaptation and its regulation. Scand J Gastroenterol Suppl. 1982;74:53–74. [PubMed] [Google Scholar]
- Garrido A. B., Jr, Freeman H. J., Chung Y. C., Kim Y. S. Amino acid and peptide absorption after proximal small intestinal resection in the rat. Gut. 1979 Feb;20(2):114–120. doi: 10.1136/gut.20.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossrau R. Conventional techniques for membrane-bound enzymes. Ciba Found Symp. 1979;(73):67–80. doi: 10.1002/9780470720561.ch5. [DOI] [PubMed] [Google Scholar]
- Gossrau R. Localization of glycoidases with naphthyl substrates. Histochem J. 1976 May;8(3):271–282. doi: 10.1007/BF01003816. [DOI] [PubMed] [Google Scholar]
- Grönqvist B., Engström B., Grimelius L. Morphological studies of the rat small intestine after jejuno-ileal transposition. Acta Chir Scand. 1975;141(3):208–217. [PubMed] [Google Scholar]
- Gutschmidt S., Kaul W., Riecken E. O. A quantitative histochemical technique for the characterisation of alpha-glucosidases in the brush-border membrane of rat jejunum. Histochemistry. 1979 Sep;63(1):81–101. doi: 10.1007/BF00508014. [DOI] [PubMed] [Google Scholar]
- Hagemann R. F., Sigdestad C. P., Lesher S. A quantitative description of the intestinal epithelium of the mouse. Am J Anat. 1970 Sep;129(1):41–51. doi: 10.1002/aja.1001290104. [DOI] [PubMed] [Google Scholar]
- Hewitt J. E., Smith M. W. Thyroid hormone effects on lactase expression by rat enterocytes. J Physiol. 1986 Jul;376:253–265. doi: 10.1113/jphysiol.1986.sp016152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I. S., Paterson J. Y., Peacock M. A., Smith M. W., Syme G. Effect of diet upon enterocyte differentiation in the rat jejunum. J Physiol. 1983 Nov;344:465–481. doi: 10.1113/jphysiol.1983.sp014952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lojda Z., Slabý J., Kraml J., Kolínská J. Synthetic substrates in the histochemical demonstration of intestinal disaccharidases. Histochemie. 1973 Mar 26;34(4):361–369. doi: 10.1007/BF00306308. [DOI] [PubMed] [Google Scholar]
- Lund E. K., Smith M. W. Rat jejunal disaccharidase activity increases biphasically during early post-natal development. J Physiol. 1987 Oct;391:487–497. doi: 10.1113/jphysiol.1987.sp016751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Menge H., Robinson J. W. Functional and structural characteristics of the rat intestinal mucosa following ileo-jejunal transposition. Acta Hepatogastroenterol (Stuttg) 1978 Apr;25(2):150–154. [PubMed] [Google Scholar]
- Menge H., Robinson J. W. The relationship between the functional and structural alterations in the rat small intestine following proximal resection of varying extents. Res Exp Med (Berl) 1978 Jul 24;173(1):41–53. doi: 10.1007/BF01851373. [DOI] [PubMed] [Google Scholar]
- Menge H., Sepúlveda F. V., Smith M. W. Cellular adaptation of amino acid transport following intestinal resection in the rat. J Physiol. 1983 Jan;334:213–223. doi: 10.1113/jphysiol.1983.sp014490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijke R. P., Hanson W. R., Plaisier H. M. The effect of transposition to jejunum on epithelial cell kinetics in an ileal segment. Cell Tissue Kinet. 1977 Jul;10(4):399–406. doi: 10.1111/j.1365-2184.1977.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]