Supplemental Digital Content is Available in the Text.
Testosterone may have a minor role in experimental pain sensitivity.
Keywords: Androgens, Testosterone, Quantitative sensory testing, Pain modulation, Experimental pain sensitivity, Sex hormones
Abstract
Animal studies have shown androgens, especially testosterone, may have an analgesic effect on nociceptive behavior. However, it is unclear if this effect is present in humans. This review and meta-analysis aim to summarize and synthesize the role of androgens on experimental pain sensitivity in humans. Studies were included if they examined the (1) relationships between androgens and experimental pain sensitivity, (2) group differences in androgen or pain levels, and (3) the effect of androgen interventions on experimental pain sensitivity. After a comprehensive search, 31 papers were identified. When possible, meta-analyses were performed. Most studies examined the impact of testosterone on experimental pain, and only a few studies focused on other androgens, such as dehydroepiandrosterone and dehydroepiandrosterone sulfate. Overall, the current data do not support the effect of androgens on experimental pain sensitivity in adult men and women with or without chronic pain. In addition, meta-analyses of Pearson correlations did not find relationships between testosterone levels and pain ratings of heat stimulus (3 studies, n = 93, Z correlation coefficient = −0.43, confidence intervals [−1.50, 0.64]) or electrical pain thresholds (4 studies, n = 147, Z correlation coefficient = 0.24, confidence intervals [−0.10, 0.58]). Moreover, contradicting results were found in intervention studies that increased or decreased testosterone levels. Thus, it is suggested that the role of testosterone on experimental pain sensitivity may be minor, even though there is a wide heterogeneity between studies. Future studies should examine the impact of other androgens and the interaction between testosterone and other hormones on experimental pain sensitivity.
1. Introduction
Sex differences in chronic and experimental pain are well documented, with higher experimental pain sensitivity in women.8,12,23,34,43,58,59 Many possible explanations for the sex differences have been suggested, including the difference in sex hormone levels.1,18,19 Interestingly, most studies have focused on the ovarian hormones, estrogen and progesterone, while the role of androgens in pain has been less studied, especially in humans. Androgens are a group of sex hormones that play a key role in sexual differentiation, reproductive health and behavior, and body development and maintenance by binding to androgen receptors.44 Androgen secretion is under neuronal control, primarily by the hypothalamus and the pituitary gland.44 Testosterone is the most studied androgen and animal studies have found that lower testosterone levels are associated with higher nociceptive responses in male rats after gonadectomy, while in female rats, lower nociceptive responses are found after testosterone treatment.2,3,14,25,28,72 In addition, studies that used androgens as a treatment for pain showed a reduction in chronic pain severity.22,33,37,38,60 Thus, the current notion is that testosterone has an antinociceptive effect on pain, including experimental pain sensitivity, and there is a critical need to assess this notion. Experimental pain sensitivity is assessed using quantitative sensory testing, which is a standardized, psychophysical approach employing controlled stimuli (eg, thermal, mechanical, vibratory) and potentially providing insights into mechanisms of pain processing and modulation. Thus, the present systemic review and meta-analysis could advance the understanding of how androgens modulate pain. The study aims were to systemically examine the findings on the role of androgens in experimental pain sensitivity in humans by focusing on (1) examining the relationships between testosterone and experimental pain sensitivity, (2) comparing group differences in androgen levels or experimental pain sensitivity, and (3) examining the effect of androgen medications on experimental pain sensitivity.
2. Methods
2.1. Search strategy
A comprehensive search was conducted with the assistance of a Washington University Becker Medical Librarian and was based on PRISMA guidelines and standards.53 CENTRAL, MEDLINE/PubMed, EMBASE, CINAHL, Web of Science, Scopus, ProQuest, OATD, EThOS, conference abstracts/proceeding: GreyLit, GreyNet, OpenGrey, Clinical trials registry platforms were used to search relevant studies published before May 2023. The systemic review was preregistered in PROSPERO (International prospective register of systematic reviews, #410889).
2.2. Study selection criteria
Three types of studies were included: (1) studies examining the relationships between testosterone and experimental pain sensitivity (associations), (2) studies comparing group differences in androgen levels or experimental pain sensitivity, and (3) studies examining the effect of androgen medications on experimental pain sensitivity. All studies were required to be conducted in humans, written in English, and have full-text access. Qualitative studies, retrospective studies, case reports, expert opinions, and reviews were excluded.
Experimental pain measures included pain thresholds, pain tolerance, pain modulation, and pain ratings of somatosensory stimuli, including pressure, heat, cold, electrical, ischemic, mechanical, and chemical. The androgen measures were measured levels of testosterone, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-s), androstenedione, androstenediol, and dihydrotestosterone (DHT). For androgen intervention studies, the primary mechanism of action of the intervention needed to be through acting on the androgen receptor or primally impacting testosterone, DHEA, DHEA-s, androstenedione, androstenediol, or DHT levels. Interventions in which the effect of the androgens could not be isolated from other treatments were excluded from the review (ie, when multiple interventions with different mechanisms of action were administered). The reference list of identified papers was scanned for additional potentially relevant papers. The complete list of search words is included in Supplementary 1, http://links.lww.com/PR9/A296. In brief, the androgen search words included androgens, testosterone, androstenedione, DHEA, DHEA-S, dehydroepiandrosterone, or hypogonadism, and the pain search words included pain, experimental pain, quantitative sensory testing, pain measurement, numeric rating scale, nociception, or threshold.
2.3. Data extraction
Five thousand one hundred thirty studies were identified. Three reviewers (H.N.A., G.B., and E.R.) independently screened the title and abstract to identify potentially relevant studies for inclusion using Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia). Each study was screened by at least 2 independent reviewers. The agreement rate was 88%, and conflicts were resolved by the third reviewer. After reviewing the titles and abstracts, 505 studies were included as potentially relevant, and their full text was reviewed. However, due to the broad search terms, most studies assessed clinical pain but not experimental pain or did not assess androgen levels. Thus, after reviewing the full text, 31 papers were identified and are included in this review and meta-analysis. Data are available upon request.
2.4. Quality assessment and risk of bias
To provide some measure of quality for the analyzed studies, we developed a scoring system based on previous similar meta-analyses.36,47,49,75 The studies were assessed based on study design, hormone collection and analyses, experimental pain assessments, and statistical analysis. In each category, if the study met the criteria, then a score of 1 was given, if not, a score of 0 was given, and if the information was not clear, a score of 0.5 was given. The final score was expressed as a percentage, calculated as the sum of the given points divided by the total points.
2.5. Statistical analysis
Data synthesis was conducted separately for the studies (1) examining the relationships between testosterone and experimental pain sensitivity, (2) comparing group differences in androgen levels or experimental pain sensitivity, and (3) examining the effect of androgen medications on experimental pain sensitivity. A synthesis of the data was conducted using a meta-analysis. The meta-analyses were conducted separately for each experimental pain measure. Due to the large heterogeneity between studies, only 2 meta-analyses were conducted to test the relationships between (1) testosterone and pain ratings of heat stimuli and (2) testosterone and electrical pain thresholds, and no other synthesis methods were conducted.13
For the meta-analyses, the number of participants and correlation coefficient for the relationships were collected from each study. If data were not presented in the manuscript, the authors were contacted in a request for the data. Meta-analyses were performed only when there were 3 or more studies examining the same experimental pain measure and androgen variable. For studies that examined more than 1 group, combined group data were prioritized. One study tested testosterone-pain relationships throughout the menstrual cycle (days 1, 4, 14, 22).74 In this study, day 1 data were prioritized because it was the only day that showed a significant relationship between testosterone levels and experimental pain measures (electrical pain thresholds). If the meta-analysis of this experimental pain measure was significant, day 1 data would be replaced with other days to test if it impacts the results. For studies with several repetitions of quantitative sensory testing involving an intervention (not androgen related), baseline data were prioritized. All analyses were conducted using R statistical software,73 the R meta-analysis package “metafor,”76 and R-Studio.66 DerSimonian and Laird's20 random-effects model was used to determine relationships between androgen levels and experimental pain sensitivity. We examined potential publication bias using funnel plots and Egger regression test for funnel plot asymmetry.
3. Results
Most studies examined the relationships between testosterone and experimental pain in healthy participants. This review focuses primarily on experimental pain measures (eg, pain thresholds rather than detection thresholds). A summary of the studies is presented in Table 1, and the quality assessment and risk of bias are provided in Supplemental Table 1, http://links.lww.com/PR9/A296.
Table 1.
Relationships between androgen levels and experimental pain sensitivity.
| Study | Sample | Hormone measure(s) | Pain measure(s) | Results | Comments |
|---|---|---|---|---|---|
| A. Testosterone | |||||
| Fillingim et al., 199724 | 11 healthy women (age 30.4, range 22–40) | Testosterone (plasma, analyzed using radioimmunoassay) | Heat and ischemic pain thresholds and tolerance applied to the arm, pain intensity ratings of heat and ischemic stimuli | No associations | Correlations were tested separately for 3 phases of the menstrual cycle (mid-follicular, ovulatory, and mid-to-late luteal) and combining all phases Pearson correlation |
| Kerem et al., 200235 | 41 healthy women in the follicular phase (age 33.0 ± 6.28) | Testosterone (plasma) | Pressure pain thresholds and tolerance | Correlation between testosterone levels, and pressure pain thresholds and tolerance (pronociceptive for pressure pain tolerance, medium effect size) | The correlation between testosterone and pressure pain thresholds is presented as negative in the text but positive in the table |
| Okifuji and Turk, 200652 | 74 women with fibromyalgia (age 29.16 ± 6.42) 74 healthy women (age 27.84 ± 6.26) |
Total testosterone (blood) | Ischemic pain threshold (arm) | Fibromyalgia Positive relationship between testosterone levels and ischemic pain thresholds in the mid-luteal phase (antinociceptive, small effect size) No associations during late follicular or perimenstrual phases Healthy No associations |
No differences in testosterone levels between women with and without fibromyalgia Pearson correlation |
| Alstergren et al., 20104 | 16 healthy men (median age 30) 36 healthy women (median age 25) |
Testosterone serum | Pressure pain threshold (glabella) and pain intensity before and after glutamate injections to the temporomandibular joint with and without N-methyl-d-aspartate receptors block | No associations | Spearman correlation |
| Teepker et al., 201074 | 32 healthy women (age = 27.3 ± 6.1, only 24 completed the study) | Free testosterone (saliva analyzed using ELISA) | Electrical, cold, and warm detection thresholds; cold, heat, pressure, and electrical pain thresholds | Positive correlation between testosterone levels and electrical pain thresholds on day 1 of the menstrual cycle (antinociceptive, medium effect size) No other associations between testosterone levels and detection or pain thresholds on day 1, 4, 14, or 22 of the menstrual cycle |
Pearson correlation |
| Ribeiro-Dasilva et al., 201164 | 35 women taking oral contraceptives (age 22.11 ± 2.78) 28 women not taking oral contraceptives (age 24.75 ± 6.73) |
Total testosterone (serum analyzed using immunoassay) | Mean heat z-score (mean of heat pain threshold, heat pain tolerance, pain ratings at 49 and 52°C), mean pressure z-score (mean of PPT at the masseter, trapezius, and ulna), mean ischemic z-score (mean of ischemic pain threshold, ischemic pain tolerance, summed intensity, and unpleasantness ratings) | No associations (tested for women with or without oral contraceptives, at the follicular or luteal stages) | The impact of testosterone levels on opioid analgesia was also examined: For women not taking oral contraceptives, which were at the luteal phase, higher testosterone was related to lower morphine analgesia for heat pain For women taking oral contraceptives, which were at the luteal phase, higher testosterone was related to greater pentazocine analgesia on ischemic pain. For women at the follicular phase, higher testosterone was associated with greater morphine analgesia on pressure pain. Pearson correlation |
| Choi et al. 201215 | 43 healthy men (age 26.2 ± 3.2) | Testosterone (saliva, collected between 14:30 and 16:30) | Electrical pain thresholds (wrist) | Positive correlation between testosterone and pain thresholds (controlling for cortisol) (antinociceptive, small effect size) | Partial correlation analysis |
| Rezaii et al., 201261 | 36 healthy women in the early follicular phase 25 healthy women in the ovulation phase 35 healthy women in the mid-luteal phase (age for all participants 25.3 ± 4.2) |
Testosterone (serum analyzed using radioimmunoassay) | Conditioned pain modulation (conditioning stimulus was cold pressor, 3°C; test stimulus was pain intensity to suprathreshold pressure) (1.2 × pressure pain thresholds) | No associations | Pearson correlation |
| Rhudy et al., 201363 | 40 healthy women (age 29 ± 8.57) | Unbound testosterone (saliva, analyzed using immunoassay) | Emotional modulation of electric pain or the nociceptive flexion reflex | No associations | Emotional modulation by viewing pictures with mutilation, neutral, and erotic contents No interaction with the menstrual phase on the relationships between testosterone and pain Mixed effect regression models |
| Vincent et al., 201378 | 12 women using combined oral contraceptive pill 12 controls |
Total testosterone (serum, analyzed using microparticle enzyme immunoassay) | The temperature that evoked pain at the intensity of 5/10 (PAIN5) applied to the left inner arm, pain intensity and unpleasantness ratings of the PAIN5 temperature (10 stimuli, 3 s duration, interstimulus interval of 55–65 s) | No associations | Correlations were tested separately for the 2 groups and when the groups were combined Pearson partial correlations |
| Bartley et al., 20159 | 40 healthy women (age 28.98 ± 8.57) | Unbound testosterone (saliva, analyzed using immunoassay) | Nociceptive flexion reflex threshold, electrocutaneous pain threshold and tolerance to the sural nerve, ischemic pain threshold and tolerance, sensory and affective ratings of electrocutaneous and ischemic pain tolerance | Higher levels of testosterone were associated with higher ischemic pain tolerance and lower electrocutaneous and ischemic sensory and affective ratings (antinociceptive) | The interaction between testosterone and the menstrual phase found that testosterone was negatively associated with electrocutaneous pain ratings during the mid-follicular, ovulatory, and late-luteal phases; however, the antinociceptive effect was strongest during the ovulation phase Linear mixed effect regression |
| Bartley et al., 201510 | 14 healthy regularly cycling women with premenstrual dysphoric disorder (age 31.1 ± 8.6) 14 healthy, regularly cycling control women without premenstrual dysphoric disorder (age 29.3 ± 7.4) |
Unbound testosterone (saliva, analyzed using immunoassay) | Nociceptive flexion reflex threshold, electrocutaneous pain threshold and tolerance to the sural nerve, ischemic pain threshold and tolerance, sensory and affective ratings of electrocutaneous and ischemic pain tolerance | All participants—testosterone was positively related to electrocutaneous pain threshold and tolerance, ischemia pain tolerance, and negatively related to affective ratings of electrocutaneous stimuli (antinociceptive, small effect size) Premenstrual dysphoric disorder group—testosterone was positively related to electrocutaneous pain threshold (antinociceptive, small effect size) Control group—no associations |
Pearson partial correlations |
| Máximo et al., 201540 | 89 women using progesterone-only contraceptives (age 28.9 ± 5.6) 99 women using combined hormonal contraceptives (age 26.4 ± 6.3) 89 women not using contraceptives (age 28.1 ± 6.7) |
Free testosterone (serum analyzed using chemoluminescence) | Pressure pain thresholds (applied to the abdominal wall and forearm) | No association | Spearman correlation coefficient |
| Choi et al., 201616 | 24 healthy (12 men, age 22.5 ± 3.9) | Testosterone (blood, analyzed using the COBRA 5010 Quantum g-counter) | Noxious heat (10 stimuli 45°C, 15 s duration) with and without transcutaneous electrical nerve stimulation | Testosterone levels were negatively correlated with average pain rating in the pain without (antinociceptive, large effect size) and pain with transcutaneous electrical nerve stimulation conditions (antinociceptive, medium effect size) | The testosterone/cortisol ratio was also calculated. The testosterone/cortisol ratio was significantly negatively correlated with the average pain rating in both conditions (medium effect size) Pearson correlation |
| Apkhazava et al., 20185 | 32 healthy men (age 21.7 ± 2.4) | Free testosterone (serum, collected in the morning, analyzed using ELISA) | Heat and cold detection and pain thresholds applied to the volar forearm | Positive correlation between testosterone levels and heat and cold pain thresholds (antinociceptive) No associations between cold/heat detection and testosterone levels |
The analysis involved comparing 3 groups of participants categorized into small, medium, and large based separately on each of the 4 sensory measures (heat/cold detection/pain thresholds) ANOVA, F score |
| Vincent et al., 201877 | 12 women | Total testosterone (serum, analyzed using a microparticle enzyme immunoassay) | The temperature that evoked pain at the intensity of 5/10 (PAIN5) applied to the left inner arm, pain intensity, and unpleasantness ratings of the PAIN5 temperature (10 stimuli, 3 s duration, interstimulus interval of 55–65 s) | Across the high estradiol data sets, testosterone levels were related to the PAIN5 temperature (lower temperatures were required with higher testosterone levels, pronociceptive, medium effect size) | Each participant completed 3 study visits, and 21 data sets were selected for analysis based on estrogen and progesterone levels Pearson partial correlations |
| Pogatzki-Zahn et al., 201956 | 15 healthy women (age 24.6 ± 4.0) | Testosterone (blood) | Experimental pain around the incision and at a control site: mechanical, cold, and warm detection threshold, mechanical, cold, and heat pain threshold; dynamic mechanical allodynia; mechanical pain sensitivity, paradoxical heat sensation, thermal sensory limen, wind-up ratio, and pain ratings to incision (volar forearm) | Pain ratings to mechanical pain were negatively related to testosterone levels in normal skin (antinociceptive, medium effect size) No associations with cold detection threshold, cold pain threshold, warm detection thresholds, heat pain threshold, wind-up ratio, mechanical pain thresholds, and incision pain as well as mechanical pain after incision |
Multiple regression (partial r) |
| Poli-Neto et al., 201957 | 26 healthy women nonusers of hormone replacement therapy (age 56.0 ± 6.7) 52 healthy women users of hormone replacement therapy (56.4 ± 5.8) 26 taking 1 mg estradiol plus 0.15 mg norethisterone acetate (age 54.9 ± 5.5) 26 taking 2.5 mg tibolone (age 57.8 ± 6.2) |
Free testosterone (serum collected around 10 am, analyzed using chemoluminescence) | Electrical pain thresholds at the flexor muscle, pressure pain thresholds at the forearm and right and left abdomen | No associations | Correlations were tested in the combined group of all participants with and without hormone replacement therapy Pearson correlation |
| Hellman et al., 202132 | 40 women with dysmenorrhea (age 22.5) 37 women with dysmenorrhea and increased bladder sensitivity (age 22) 18 women with bladder pain syndrome (age 30.5) 29 healthy controls (age 23) |
Testosterone (serum, analyzed using enzyme immunosorbent assays) | Bladder pain after water load test (drinking 20 ounces of water within 5 min), pelvic pain pressure applied transvaginally and at the trapezius, medial knee fat pad, greater trochanter, and forehead, cold pain ratings (immersion of the right hand in cold water) | No associations | Participants who had testosterone concentrations below the assay's sensitivity range were assigned a concentration of 10 ng/dL No difference in testosterone across groups Spearman partial correlation coefficients |
| Vollert et al., 202279 | 62 healthy participants (n = 30 women, median age 24 y) | Testosterone (serum, analyzed using immunoassay) | Conditioned pain modulation responses were calculated using early and late paradigms, offset analgesia constant, dynamic offset effect, and offset control were calculated | Men Testosterone explained between 0% and 7% of pain measures variance Women Testosterone explained between 0% and 11% of pain measures variance Women (luteal) Testosterone explained between 0% and 6% of pain measures variance Women (follicle) Testosterone explained between 2% and 15% of pain measures variance Women (ovulation) Testosterone explained between 2% and 17% of pain measures variance In a multiple regression model correcting for sex, pain catastrophizing, and stimulus temperature, testosterone had an effect only on the early CPM |
Correlations were calculated separately for men and women and also for women in luteal, follicle, and ovulation P values are not indicated for the correlations Pearson correlation Test stimulus (conditioned pain modulation paradigm) and T1-temperature (offset analgesia paradigm) were between PAIN55 and PAIN65 |
| Zhang et al., 202384 | 66 healthy women (age 25.91 ± 1.83) | Testosterone (plasma collected at 4 pm, analyzed using microparticle chemiluminescence immune analyzer) | The change in pressure, cold and ischemic pain thresholds and tolerance and pinprick evoked pain between the early to mid-follicular subphase and the mid-luteal subphase | No associations between the change in testosterone and the change in pain between the early to mid-follicular phase and the mid-luteal phase | Pearson correlation |
| Pan et al., 202454 | 75 patients with migraine (30 men, age: 31.1 ± 7.7) 88 healthy controls (41 men, age: 29.9 ± 7.7) |
Free testosterone (saliva, collected between 9:00 and 15:00, analyzed using enzyme-linked immunosorbent assay) | Heat pain threshold and pain rating to 45°C | Healthy controls No correlations between heat pain thresholds and testosterone levels across men, women, or the combined group Higher testosterone levels were related to higher heat pain ratings in men (pronociceptive, medium size effect), but higher testosterone levels were related to lower heat pain ratings in women (antinociceptive, medium size effect) Patients with migraine No associations in men, women, or the combined group |
No direct comparison between patients and controls Pearson correlation |
| B. DHEA and DHEA-S | |||||
| Freitas et al., 201226 | 17 pos-menopausal women with fibromyalgia (53 ± 7.98) 19 healthy postmenopausal women (53.32 ± 6.46) |
DHEA-S (plasma collected between 8:00 and 9:30 am, ed using immunoassay) | Pressure pain threshold and tolerance | Fibromyalgia patients: positive correlations between DHEA-S levels and pain threshold and tolerance (antinociceptive, small size effect) | DHEA-S levels were not different between the groups Generalized linear model |
| Yamamotova et al., 201281 | 20 women with anorexia nervosa and taking hormonal replacement therapy (6 binge/purging type and 14 restrictive type, age 24.8 ± 4.6) 21 healthy women (age matched) |
DHEA, DHEA-S (blood collected in the morning hours) | Heat pain threshold latency | Patients Negative correlation between DHEA and heat pain threshold (pronociceptive, medium size effect) No association with DHEA-S Healthy No association Whole sample Negative correlation between DHEA-S and heat pain threshold (pronociceptive, medium size effect) No association with DHEA |
The cortisol/DHEA and cortisol/DHEA-S ratios were also tested and positive associations between the ratios and heat pain thresholds were found only in the patient group Pearson correlation |
| Scioli-Salter et al., 201670 | 5 trauma-exposed participants with comorbid chronic pain/posttraumatic stress disorder 7 trauma-exposed participants without comorbid chronic pain/posttraumatic stress disorder (age for all participants 39.0 ± 10.3) |
DHEA (plasma, collected between 8:00 and 12:00, analyzed using radioimmunoassay) | Pain tolerance using the cold pressor test (4°C) before and after peak cardiopulmonary exercise testing | The change in DHEA before vs after exercise was negatively correlated with pain tolerance after exercise (medium effect size) | DHEA levels were not different between groups Pearson correlation |
| C. Group differences | |||||
| Choi et al. 201717 | 26 healthy men divided based on their testosterone levels High testosterone (n = 13, age 23.08 ± 2.3) Low testosterone (n = 13, age 22.15 ± 2.41) |
Testosterone (blood, analyzed using the COBRA 5010 Quantum g-counter) | Pain intensity and unpleasantness to noxious heat stimuli (30 s, finger immersion at 50°C repeated 5 times) | Participants with low testosterone levels had higher pain ratings compared with participants with high testosterone levels (antinociceptive) | t test |
| Archey et al., 20196 | 46 participants (32 women, age ± SE 21.61 ± 0.55) Women were divided into individuals who did (n = 23) and did not (n = 9) display behaviors indicating pain during cold pressor test |
Testosterone (saliva collected between 11:00 and 19:00, analyzed using enzyme-linked immunosorbent assay) | Pain ratings at the cold pain thresholds and tolerance (cold pressor test, 2°C, hand) | No differences in testosterone levels at baseline, after CPT or the change from baseline to after CPT between the women subgroups although women who did display behaviors indicating pain had higher pain ratings | t test |
DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate.
3.1. Relationships between testosterone and experimental pain sensitivity
3.1.1. Thermal stimuli
A significant positive correlation was found between heat pain thresholds and free testosterone levels in healthy men (ie, higher levels of testosterone are associated with a higher pain threshold). This supports the antinociceptive effect of testosterone in healthy men. However, this study found no significant correlations between cold/heat detection and free testosterone levels.5 Another study similarly found an antinociceptive effect such that higher testosterone levels were correlated with lower pain ratings to noxious heat stimuli delivered alone (large effect size) or with transcutaneous electrical nerve stimulation (medium effect size) in healthy men and women.16 However, higher testosterone levels in healthy women were associated with lower temperatures that induced pain at the intensity of 50 (0–100 scale, medium effect size), suggesting a pronociceptive effect of testosterone.77 Another study in healthy women found no correlation between testosterone levels and pain thresholds, pain tolerance, and pain intensity ratings to heat stimuli.24 Interestingly, sex differences in the relationships between testosterone and heat pain ratings were also reported, and while no associations were found when examining the entire cohort when analyzing by sex, a positive medium size correlation was found in men (suggestive of pronociceptive effect) and a negative medium size correlation in women suggestive of antinociceptive effect.54
One challenge when assessing sex hormone levels in women is the role of the menstrual cycle and medications that can impact sex hormone levels, such as contraceptive pills and hormone replacement therapy. Testosterone levels and experimental pain sensitivity were examined in healthy women on days 1, 4, 14, and 22 of their menstrual cycle. Overall, in any of those days, no correlations were found between free testosterone levels and heat detection and heat pain thresholds.74 In addition, the change in testosterone levels between the early-mid follicular and mid-luteal phases did not correlate with the change in pain sensitivity to cold stimuli.84 In women with and without oral contraceptives, testosterone levels did not correlate with heat pain sensitivity (the temperature that induced pain at the intensity of 50 [0–100 scale] and the pain intensity and unpleasantness ratings of this temperature).64,78
In patients with chronic pain, no relationship between testosterone levels and provoked pain has been identified. In men and women with migraine, there was no correlation between testosterone levels and heat pain thresholds or pain ratings to heat stimuli.54 Similarly, in healthy women without chronic pain other than dysmenorrhea and/or bladder sensitivity, there was no correlation between testosterone levels and pain ratings to cold water stimulus.32
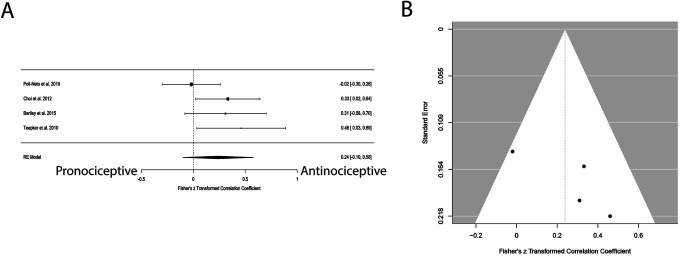
A meta-analysis was conducted between testosterone levels and pain ratings of heat stimuli. Three studies were included in the meta-analysis. Even though 2 reported on a medium-large antinociceptive effect of testosterone, overall, the meta-analysis found no evidence of a significant correlation between these factors (Fig. 1A, Table 2), and there was no indication of publication bias (Fig. 1B, Table 2). Importantly, 2 of the included studies assessed pain ratings to heat stimuli, which was set to evoke pain at an intensity of 5/10; thus, the variability in pain ratings in these studies is, by design, small.77,78 However, this measure can provide additional insight into pain sensitivity to heat stimuli.
Figure 1.
Forest and funnel plots for the association between testosterone levels and heat pain. (A) No correlation between testosterone levels and heat pain ratings across the studies. Importantly, in the studies by Vincent et al., participants rated their pain intensity to stimuli that were tailored to evoke an intensity of 50 (0–100 scale); thus, lower variability in these studies is expected for pain ratings. (B) No indication of publication bias.
Table 2.
Summary of tests for heterogeneity, grand mean, and publication bias.
| Testosterone and pain test | No. included studies (n participants) | P | τ 2 | τ | I2 (%) | H2 | Cochran Q test for heterogeneity | Grand mean correlation r (95% Cl) in original units | Egger regression test for funnel plot asymmetry (P) |
|---|---|---|---|---|---|---|---|---|---|
| Pain ratings of heat stimulus | 3 (84) | 0.227 | 0.173 (SE = 0.216) | 0.416 | 81.46 | 5.39 | Q (df = 2) = 10.785, P = 0.005 | r = −0.404 (−0.904 to 0.565) | P = 0.377 |
| Electrical pain thresholds | 4 (147) | 0.111 | 0.018 (SE = 0.040) | 0.133 | 36.41 | 1.57 | Q (df = 3) = 4.717, P = 0.194 | r = 0.234 (−0.100 to 0.521) | P = 0.191 |
H2, total variability/within-study variance; I2, % of total variability due to heterogeneity; t, square root of t2; t2, estimate of the total amount of heterogeneity.
3.1.2. Electrical stimuli
Several studies reported an antinociceptive effect of testosterone on electrical pain sensitivity. In healthy men, a positive small effect size correlation between testosterone levels and electrical pain thresholds was found, suggesting an antinociceptive effect of testosterone.15 Similarly, in healthy women, higher levels of testosterone were associated with lower electric sensory and affective pain ratings.9 The same group also found a small antinociceptive effect for a combined group of women with premenstrual dysphoric disorder and healthy controls, in which testosterone levels were positively related to electrocutaneous pain thresholds and electrocutaneous pain tolerance and negatively correlated with affective pain ratings of electrocutaneous stimuli.10 Contradictory to this, free testosterone levels were not related to electrical pain thresholds in healthy postmenopausal women receiving and or not receiving hormone replacement therapy.57 In addition, another study assessing a different type of electrical stimulus, which is nonpainful and represents sensory detection (not pain), found that free testosterone levels were not related to electrical detection thresholds in healthy women tested on days 1, 4, 14, and 22 of their menstrual cycle.74 However, this study found an antinociceptive medium size effect of testosterone on electrical pain thresholds only on day 1 of the menstrual cycle.74
Four studies were included in the meta-analysis. Even though 3 reported on a small-medium antinociceptive effect of testosterone, overall, the meta-analysis revealed no evidence of a significant correlation between testosterone levels and electrical pain thresholds across the studies (Fig. 2A, Table 2). There was no indication of publication bias (Fig. 2B, Table 2).
Figure 2.
Forest and funnel plots for the association between testosterone levels and electrical pain thresholds. (A) No correlation between testosterone levels and electrical pain thresholds across the studies. (B) No indication of publication bias.
3.1.3. Ischemic stimuli
In healthy women, higher levels of testosterone were associated with higher ischemia tolerance and lower ischemia sensory and affective pain ratings.9 Similarly, in women with premenstrual dysphoric disorder and healthy controls, testosterone levels were positively correlated to ischemia pain tolerance, which suggested an antinociceptive effect, although the effect size was small.10 However, other studies found no correlations between testosterone levels and ischemic pain sensitivity for women with and without oral contraceptives in the follicular or luteal phases.64 Testosterone was also not related to pain thresholds, pain tolerance, and pain intensity ratings of ischemic stimuli.24,52 In addition, the changes in testosterone levels between the early-mid follicular and mid-luteal phases were not related to the changes in ischemic pain sensitivity.84 In patients with fibromyalgia, a positive relationship of small size effect between total testosterone levels and ischemic pain thresholds was found, suggesting an antinociceptive effect of testosterone. However, this relationship was found only in the mid-luteal phase and not during the late follicular or perimenstrual phases.52
A meta-analysis was not performed because there were <3 studies with complete data for the same experimental ischemic pain measure and androgen variable.
3.1.4. Pressure stimuli
Overall, no relationships were found between testosterone levels and pressure pain sensitivity. In both healthy men and women, testosterone levels were not related to pressure pain thresholds (PPTs).4 Similar results were found in healthy women tested 4 times across their menstrual cycle74 and healthy women with and without contraceptive pills.40,57,64 No correlations were found between the change in testosterone levels and the change in pain sensitivity to pressure stimuli between the early-mid follicular and the mid-luteal phase.84 Notably, 1 study found a pronociceptive effect (medium size effect) of testosterone, which was negatively related to PPT and pressure pain tolerance in healthy women in the follicular phase.35 Only 1 study tested these associations in patients with chronic pain conditions. No relationships between testosterone levels and pressure pain thresholds were found for a combined group, which included women with dysmenorrhea, women diagnosed with bladder pain syndrome, women with dysmenorrhea and increased bladder sensitivity, and healthy pain-free controls.32
A meta-analysis was not performed because there were <3 studies with complete data for the same experimental pressure pain measure and androgen variable.
3.1.5. Other stimuli
For mechanical pinprick stimuli, testosterone levels were negatively correlated to mean pain ratings (medium antinociceptive effect) but were not related to mechanical pain thresholds, incision pain, or mechanical temporal summation.56 In addition, the change in testosterone levels was not related to the change in pain sensitivity to pinprick stimuli between the early-mid follicular and mid-luteal phases.84 Testosterone levels were not related to pain intensity ratings using a paradigm of glutamate injections into the temporomandibular joint.4 Finally, no relationships were found between testosterone levels and bladder pain evoked by drinking water in a combined group of healthy women without chronic pain other than dysmenorrhea and/or bladder pain syndrome.32
3.1.6. Pain modulation
Similar to experimental pain sensitivity, overall, testosterone did not affect inhibitory pain modulation capabilities using various pain modulation paradigms. One of the pain modulation paradigms that were tested was the conditioned pain modulation (CPM) paradigm, which assesses the “pain inhibits pain” phenomenon. In this paradigm, 1 noxious stimulus is used to inhibit the pain evoked by another noxious stimulus delivered to a remote area in the body.45,48,50,82 The 2 stimuli can be delivered simultaneously (parallel) or one after the other (sequential).46 The exact mechanism of CPM is not known but may involve descending modulation, propriospinal inhibition and, supraspinal mechanisms.48,50 In women in early follicular, ovulation, or mid-luteal phases, no correlations between testosterone levels and CPM responses were found.61 In healthy men and women, testosterone had no effect on the sequential CPM response or offset analgesia but a significant effect on the parallel CPM response in a multiple regression model correcting for sex, catastrophizing, and stimulus temperature. However, in this study, testosterone levels had a very small effect and explained less than 6% of the variance in CPM responses (parallel or sequential, conducted separately for men and women, and for women divided based on luteal, follicle, and ovulation).79
Another study in healthy women used a different pain modulation paradigm of emotional pain modulation.63 The inhibitory mechanisms underlying emotional pain modulation may differ from the mechanisms of CPM and include supraspinal regions involved in emotional or cognitive processing.63 Testosterone levels and the interaction between testosterone and menstrual phases were not related to the emotional modulation of pain ratings or nociceptive flexion reflex to electrical stimuli during the viewing of pictures containing mutilation, neutral, or erotic contents.63
3.2. Relationships between dehydroepiandrosterone, dehydroepiandrosterone sulfate, and experimental pain
A few studies focused on DHEA and DHEA-S, which are involved in testosterone synthesis. A summary of these studies is presented in Table 1B. In patients with trauma but no chronic pain and patients with chronic pain/posttraumatic stress disorder, a greater increase in DHEA after exercise was correlated with a decrease in cold pain tolerance, suggesting a pronociceptive medium size effect of DHEA.70 In women with anorexia nervosa, heat pain threshold latency correlated negatively with DHEA (higher DHEA associated with higher pain, medium size effect), supporting a pronociceptive effect of DHEA.81 However, this was found only in the patient group and not in the control group. When patients and controls were combined, a significant pronociceptive medium size effect was found (higher DHEA-S was associated with higher heat pain threshold latency).81 By contrast, in a study of patients with fibromyalgia, a positive correlation was found between DHEA-S levels and pressure pain thresholds and tolerance and, although the effect size was small, it indicates an antinociceptive effect of DHEA-S.26 Thus, DHEA and DHEA-S have been studied less, and published studies have shown contradictory results on their role in experimental pain sensitivity.
3.3. Group differences
A summary of the studies is presented in Table 1C. Healthy men were divided based on their normal variability in testosterone levels. Men with low testosterone levels had higher pain intensity and unpleasantness ratings to a heat stimulus compared with men with high testosterone levels (large effect size for both pain intensity and unpleasantness ratings).17 Using a different approach, healthy women were divided into women who displayed/did not display pain behaviors during a cold pressor test (based on the experimenter's judgment, which noted any physical or vocal display of discomfort and divided the participants accordingly). Women who displayed pain behaviors had higher pain scores on the cold pressor test compared with women who did not display pain behaviors. However, no significant differences in testosterone levels were found between the groups at baseline, after the cold pressor test, or in the change from baseline to after the cold pressor test.6
3.4. The effect of androgen medications on experimental pain sensitivity
All studies have examined testosterone-related interventions. A summary of the studies is presented in Table 3. In healthy men, the effect on the experimental pain sensitivity of a one-time testosterone gel application was compared with placebo. Men who received the testosterone had higher pain intensity and unpleasantness ratings to noxious and nonnoxious electrocutaneous stimuli compared with placebo, suggesting a pronociceptive effect of testosterone gel on both pain and somatosensory stimuli in general.85 Other studies examined the effect of testosterone on experimental pain in patients who experienced changes in testosterone levels due to medications. For example, a side effect of opioids is hypogonadism (reduced levels of testosterone), which can be managed with testosterone treatment. In patients with chronic pain using opioids, a reduction in punctate mechanical pain ratings and an increase in pressure pain threshold at the thumb were found in patients receiving testosterone treatment compared with placebo. Cold pain tolerance, cold pain ratings, and pressure pain thresholds at the trapezius were not different between the groups.11 In another study with the same patient population, testosterone injections and placebo had similar effects, and no differences were found in the change from baseline to 6-month follow-up in pressure pain thresholds, heat pain thresholds, cold pain thresholds, pressure pain tolerance, temporal summation, and the conditioned pain modulation response.29 Another group of patients who have significant changes in testosterone levels are patients with prostate cancer, which is treated with androgen deprivation therapy to reduce testosterone levels. A comparison between patients with androgen deprivation therapy and a control group of patients with prostate cancer in remission found no significant group differences in pressure pain thresholds, pain ratings of cuff pressure algometry and cold pain, mechanical temporal summation, and conditioned pain modulation responses.27 Overall, these interventional studies found no effect of testosterone manipulations on experimental pain sensitivity.
Table 3.
Effect of androgen nterventions on experimental pain sensitivity.
| Study | Sample | Study design | Hormone intervention and/or measure | Pain measure(s) | Results | Comments |
|---|---|---|---|---|---|---|
| Basaria et al. 201511 | Men with chronic noncancer pain with opioid-induced androgen deficiency 36 in the testosterone group (age 48 ± 9) 29 in the placebo group (age 50 ± 6) |
Randomized, double-blind, parallel placebo-controlled trial | 5 g of a transdermal testosterone gel or placebo gel, applied once daily for 3 mo | Changes in pressure pain thresholds (trapezius, thumb), cold pain tolerance, pain ratings to cold and mechanical pinprick pain before vs after the intervention | The testosterone group had an increase in pressure pain threshold at the thumb and a reduction in mechanical pain ratings compared with the placebo group (antinociceptive) No differences in cold pain tolerance and ratings and pressure pain thresholds at the trapezius |
Brief Pain Inventory was also assessed, but no differences were found between the testosterone and placebo groups |
| Gagliano-Juca et al., 201827 | Men with prostate cancer and with no chronic pain 37 men in the androgen deprivation therapy group (age 67 ± 8) 40 men in the nonandrogen deprivation therapy group had prostatectomy and/or radiation therapy at least 6 months before enrollment and were in remission (66 ± 7) |
Prospective observational cohort study | Androgen deprivation therapy was GnRH agonist (22.5 mg of leuprolide acetate) every 3 months for at least 6 months and androgen receptor antagonist (bicalutamide) during the first month | Pressure pain thresholds, pain ratings of pressure and cold pain, mechanical temporal summation, conditioned pain modulation tested at baseline, 6 wk, 3 mo, and 6 mo | No differences between groups | Changes in Brief Pain Inventory scores did not differ between the 2 groups |
| Glintborg et al., 202029 | Men with total testosterone <12 nmol/L and treated with opioids for nonmalignant pain disease 20 in the testosterone group (age 54) 21 in the placebo group (age 55) |
Double-blind, randomized, placebo-controlled trial | Testosterone undecanoate, 1000 mg or placebo injections given at the time of randomization, and at 6 and 18 wk Total and bioavailable testosterone (collected in the morning, analyzed using liquid chromatography-tandem mass spectrometry) |
Pressure pain thresholds and tolerance, pressure temporal summation, conditioned pain modulation, heat and cold pain thresholds, assessed at baseline and after 6 mo | Changes in experimental pain sensitivity were not different between testosterone and placebo groups Higher changes in total and bio testosterone were associated with larger reductions in peak pain intensity in the testosterone group (antinociceptive) |
Testosterone treatment did not change pain ratings of clinical pain or bodily pain (SF-36 except for the SF-36 physical component score) |
| Zhuo et al., 202385 | 29 healthy men (age ± SE 20.53 ± 0.42) | Randomizer, double-blind, placebo-controlled, crossover | Single dose of 150 mg testosterone gel or placebo gel | Pain intensity and unpleasantness ratings to electrocutaneous stimuli of PAIN2 (nonpainful but a clearly detectable sensation) and PAIN6 intensity (painful but tolerable sensation) | Higher pain intensity and unpleasantness ratings in the testosterone session than in the placebo session (pronociceptive) |
4. Discussion
The present review and meta-analysis explored the impact of androgens on experimental pain sensitivity assessed using quantitative sensory testing, which is a widely used, standardized psychophysical method employing controlled stimuli to measure pain perception and specific mechanistic processes (eg, peripheral and central sensitization, inhibitory modulation, etc.) in humans.7 Overall, some studies suggest a small antinociceptive effect of androgens, others report a small pronociceptive effect, and some find no significant effects at all. Thus, testosterone may have only a minor impact on experimental pain sensitivity in men and women with or without chronic pain. This is contradictory to animal studies that found an antinociceptive and protective effect of testosterone. In animal models, a reduction in testosterone levels after orchiectomy increases nociceptive behavior, while testosterone treatment reduces nociceptive behavior.2,3,14,25,28,39,72 Importantly, these manipulations cause extreme changes in testosterone levels dramatically outside of the physiologic range. Thus, animal studies may not be comparable to human studies, which mostly assessed the natural variability of testosterone levels in healthy participants and correlated them with experimental pain sensitivity.
4.1. No relationships between androgen levels and experimental pain sensitivity
Testosterone could impact pain through several mechanisms, including affecting sensory neurons, interacting with immune cells, and impacting brain areas involved in pain processing, stress, and mood, such as anxiety and depression.21 Thus, it is surprising that no relationships were found, although only 2 meta-analyses were performed due to the large variability in the methodology of the tests. Both meta-analyses between testosterone levels and electrical pain thresholds, as well as pain ratings to heat stimuli, did not find significant relationships. There are many confounding factors that could impact testosterone levels and their relationships with pain. These factors include sex, age, menstrual phase, sample type (saliva, blood), analysis method (radioimmunoassay, liquid chromatography-tandem mass spectrometry), hormone analysis (free, total), and time of sample collection.65 Notably, not all studies controlled for these factors (Supp Table 1, http://links.lww.com/PR9/A296), which can affect their results. However, due to the small number of studies, our meta-analyses included all available studies with the same androgen and experimental pain measures, and conducting separate meta-analyses based on the above-mentioned factors was not possible. It is possible that the lack of significant associations in the meta-analyses is due to the small number of included studies, the heterogeneity of the individual studies, and their small sample and methodological limitations. Thus, testosterone may have a subtle small effect on pain, which could not be detected in our analyses. Conducting highly rigorous studies on the relationships between androgens and experimental pain is highly needed. In addition, more studies are needed to determine the role of other androgens on experimental pain in specific populations. For example, androgens may impact pain in some populations, such as in patients with chronic pain conditions in which the underlying mechanism of the condition is related to sex hormones. These conditions, such as endometriosis, vaginal pain, visceral pain, and headache, may be more hormone-responsive compared with other chronic pain conditions or experimental pain measures.30,31 Indeed, several studies found that testosterone or DHEA treatments improve pain in patients with endometriosis and vaginal pain.22,33,37,38,60 Thus, patients with these conditions may have a specific nervous system organization, allowing testosterone to induce its antinociceptive activational effect.
Testosterone is the most studied androgen, and only a few studies have focused on the other hormones involved in testosterone synthesis, mainly DHEA and DHEA-S. Dehydroepiandrosterone and dehydroepiandrosterone sulfate are secreted primarily by the adrenal cortex and are precursors to testosterone.42,44 In addition, testosterone can be converted to DHT, which has a greater affinity for the androgen receptor.44 Although less studied, there are more consistent results for a pronociceptive effect of DHEA on experimental pain sensitivity.70,81 An important next research step is a more in-depth examination of the various less-studied androgens, including DHEA, DHEA-S, DHT, and androstenedione. In addition, examining the ratio or interaction between testosterone and other sex hormones can be of interest as this interplay may have a greater impact on pain sensitivity than an isolated androgen level. For example, the ratio between testosterone and cortisol was correlated (medium effect size) to heat pain ratings in healthy men and women.16 In addition, while no differences in testosterone levels were found in patients with migraine compared with healthy controls, the testosterone/cortisol ratios were significantly lower in patients with migraine as compared with those without.55
The lack of associations between androgen levels and experimental pain sensitivity may suggest that a relatively small individual variability in the normative ranges of androgen levels is insufficient to reveal the impact of androgens on pain. It is possible that only extremely low/high values of androgen levels, such as induced following interventions, can impact experimental pain sensitivity.
4.2. Androgen interventions and experimental pain sensitivity
The few human studies that examined the impact of testosterone supplementation on experimental pain sensitivity found contradicting results. These interventions include testosterone supplementation to achieve a normal physiologic range in patients with opioid-induced hypogonadism41,51 and androgen deprivation therapy to reduce testosterone levels in patients with prostate cancer.62,83 Thus, the extreme testosterone manipulations in these populations may be more like the manipulation used in animal studies. Most of the human manipulation studies are of patients with conditions such as chronic pain requiring chronic opioids or cancer, which likely impact testosterone levels, pain sensitivity, and the relationships between them. In addition, it is also possible that androgens and testosterone may be more involved in acute or chronic pain severity rather than experimental pain sensitivity. In patients with fibromyalgia, while no relationships were found between experimental pain sensitivity and testosterone levels,52 lower testosterone levels were related to higher clinical pain,67 and testosterone treatment reduced muscle pain and tender points pain.80 Moreover, testosterone supplementation has been found to reduce clinical pain in various other conditions, including endometriosis.22,33,60 Thus, testosterone may have a greater impact on chronic pain rather than experimental pain.
4.3. Organizational or activational effects of androgens
Androgens and testosterone have organizational and activational effects. An activational effect depends on the presence of the hormone at a specific moment to impact behavior, while an organizational effect indicates a permanent change in the organization of the nervous system, which occurs when the hormone is present during specific critical periods in life such as perinatal and puberty.68,71 The organizational changes can impact the activational response and behavior later in life (ie, in adulthood).71 For example, in hamsters, testosterone administration before or during puberty but not after led to changes in mating behavior in adulthood.69 Thus, based on the early life organizational changes, there is an individual variability in the response to sex hormones, which can explain the large variability in the effect of sex hormones on behavior and pain.71 It is possible that testosterone has an activational antinociceptive effect only in individuals who had a specific organizational change, making them more responsive to testosterone effects later in life.
4.4. Strengths and limitations
This review has several strengths: (1) the protocol was preregistered in PROSPERO (#410889); (2) a comprehensive search strategy was developed with the assistance of a medical librarian and experts in pain, endocrinology, and gynecology; (3) a comprehensive literature search was conducted; (4) risk of bias was assessed; (5) screening of studies was completed by several investigators with high agreement level; and (6) results were synthesized, and meta-analyses were performed. Notably, there are several limitations: (1) the studies are heterogeneous in their methods and studied population, and thus, only 2 meta-analyses were performed; (2) a small number of studies were included in each meta-analysis; (3) an independent information specialist did not review the search strategy; and (4) many studies have biases and limitations. These limitations may explain the lack of associations found in the meta-analyses. However, overall, most individual studies reported a lack of association or no impact of androgen intervention on experimental pain. Even if only the high-quality studies were included, no consistent finding of the effect of androgens on experimental pain would be found, as conflicting results between and within studies are reported.
5. Conclusions
This review and meta-analysis summarize the current knowledge on the role of androgen on experimental pain, point out the existing gaps, and propose future directions. Overall, physiologic variability in testosterone levels may have a minimal impact on experimental pain sensitivity in adult humans with and without chronic pain. This is contradictory to the findings in animal studies. Manipulation of testosterone to extremely high or low levels may have a greater impact on pain, but only in some patient populations. Future studies should focus on examining the interactions between androgens and other hormones in specific subgroups of patients.
Disclosures
The authors declare no competing interests in relation to this work.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A296.
Acknowledgments
The authors thank Michelle Doering for her help with the literature search. This study is supported by NIH (R01NS129742 and R01NS134986). Data are available upon request.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Elizabeth Wu-Chen, Email: e.j.wu-chen@wustl.edu.
Gourav Banerjee, Email: gourav@wustl.edu.
Elise Requadt, Email: elise.requadt@wustl.edu.
Benjamin Hunter, Email: hunter.ben1@outlook.com.
Thomas J. Baranski, Email: baranski@wustl.edu.
Whitney Trotter Ross, Email: ross.w@wustl.edu.
References
- [1].Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm Behav 2006;50:1–7. [DOI] [PubMed] [Google Scholar]
- [2].Aloisi AM, Ceccarelli I, Fiorenzani P. Gonadectomy affects hormonal and behavioral responses to repetitive nociceptive stimulation in male rats. Ann N Y Acad Sci 2003;1007:232–7. [DOI] [PubMed] [Google Scholar]
- [3].Aloisi AM, Ceccarelli I, Fiorenzani P, De Padova AM, Massafra C. Testosterone affects formalin-induced responses differently in male and female rats. Neurosci Lett 2004;361:262–4. [DOI] [PubMed] [Google Scholar]
- [4].Alstergren P, Ernberg M, Nilsson M, Hajati AK, Sessle BJ, Kopp S. Glutamate-induced temporomandibular joint pain in healthy individuals is partially mediated by peripheral NMDA receptors. J Orofac Pain 2010;24:172–80. [PubMed] [Google Scholar]
- [5].Apkhazava M, Kvachadze I, Tsagareli M, Mzhavanadze D, Chakhnashvili M. The relationship between thermal pain sensation, free testosterone, TRPV1, MOR levels and various degrees of hostility in young healthy males. Georgian Med News 2018;283:109–14. [PubMed] [Google Scholar]
- [6].Archey M, Goldey K, Crockett E, Boyette-Davis J. An investigation of the effects of testosterone and behavioral expressions of pain on sex/gender differences in pain perception. Psychol Rep 2019;122:826–40. [DOI] [PubMed] [Google Scholar]
- [7].Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 2009;10:556–72. [DOI] [PubMed] [Google Scholar]
- [8].Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bartley EJ, Palit S, Kuhn BL, Kerr KL, Terry EL, DelVentura JL, Rhudy JL. Natural variation in testosterone is associated with hypoalgesia in healthy women. Clin J Pain 2015;31:730–9. [DOI] [PubMed] [Google Scholar]
- [10].Bartley EJ, Palit S, Kuhn BL, Kerr KL, Terry EL, DelVentura JL, Rhudy JL. Nociceptive processing in women with premenstrual dysphoric disorder (PMDD): the role of menstrual phase and sex hormones. Clin J Pain 2015;31:304–14. [DOI] [PubMed] [Google Scholar]
- [11].Basaria S, Travison TG, Alford D, Knapp PE, Teeter K, Cahalan C, Eder R, Lakshman K, Bachman E, Mensing G, Martel MO, Le D, Stroh H, Bhasin S, Wasan AD, Edwards RR. Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial. PAIN 2015;156:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boerner KE, Birnie KA, Caes L, Schinkel M, Chambers CT. Sex differences in experimental pain among healthy children: a systematic review and meta-analysis. PAIN 2014;155:983–93. [DOI] [PubMed] [Google Scholar]
- [13].Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J, Welch V, Thomson H. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ceccarelli I, Scaramuzzino A, Massafra C, Aloisi AM. The behavioral and neuronal effects induced by repetitive nociceptive stimulation are affected by gonadal hormones in male rats. PAIN 2003;104:35–47. [DOI] [PubMed] [Google Scholar]
- [15].Choi JC, Chung MI, Lee YD. Modulation of pain sensation by stress-related testosterone and cortisol. Anaesthesia 2012;67:1146–51. [DOI] [PubMed] [Google Scholar]
- [16].Choi JC, Kim J, Kang E, Lee JM, Cha J, Kim YJ, Lee HG, Choi JH, Yi DJ. Brain mechanisms of pain relief by transcutaneous electrical nerve stimulation: a functional magnetic resonance imaging study. Eur J Pain 2016;20:92–105. [DOI] [PubMed] [Google Scholar]
- [17].Choi JC, Park YH, Park SK, Lee JS, Kim J, Choi JI, Yoon KB, Lee S, Lim DE, Choi JY, Kim MH, Park G, Choi SS, Lee JM. Testosterone effects on pain and brain activation patterns. Acta Anaesthesiol Scand 2017;61:668–75. [DOI] [PubMed] [Google Scholar]
- [18].Craft RM. Modulation of pain by estrogens. PAIN 2007;132(suppl 1):S3–12. [DOI] [PubMed] [Google Scholar]
- [19].Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain 2004;8:397–411. [DOI] [PubMed] [Google Scholar]
- [20].DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [21].Dourson AJ, Darken RS, Baranski TJ, Gereau RW, Ross WT, Nahman-Averbuch H. The role of androgens in migraine pathophysiology. Neurobiol Pain 2024;16:100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fernandes T, Costa-Paiva LH, Pinto-Neto AM. Efficacy of vaginally applied estrogen, testosterone, or polyacrylic acid on sexual function in postmenopausal women: a randomized controlled trial. J Sex Med 2014;11:1262–70. [DOI] [PubMed] [Google Scholar]
- [23].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL, III. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fillingim RB, Maixner W, Girdler SS, Light KC, Harris MB, Sheps DS, Mason GA. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med 1997;59:512–20. [DOI] [PubMed] [Google Scholar]
- [25].Fischer L, Clemente JT, Tambeli CH. The protective role of testosterone in the development of temporomandibular joint pain. J Pain 2007;8:437–42. [DOI] [PubMed] [Google Scholar]
- [26].Freitas RP, Lemos TM, Spyrides MH, Sousa MB. Influence of cortisol and DHEA-S on pain and other symptoms in post menopausal women with fibromyalgia. J Back Musculoskelet Rehabil 2012;25:245–52. [DOI] [PubMed] [Google Scholar]
- [27].Gagliano-Jucá T, Travison TG, Nguyen PL, Kantoff PW, Taplin ME, Kibel AS, Manley R, Hally K, Bearup R, Beleva YM, Huang G, Edwards RR, Basaria S. Effects of androgen deprivation therapy on pain perception, quality of life, and depression in men with prostate cancer. J Pain Symptom Manage 2018;55:307–17.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gaumond I, Arsenault P, Marchand S. Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Res 2005;1052:105–11. [DOI] [PubMed] [Google Scholar]
- [29].Glintborg D, Vaegter HB, Christensen LL, Bendix E, Graven-Nielsen T, Andersen PG, Andersen M. Testosterone replacement therapy of opioid-induced male hypogonadism improved body composition but not pain perception: a double-blind, randomized, and placebo-controlled trial. Eur J Endocrinol 2020;182:539–48. [DOI] [PubMed] [Google Scholar]
- [30].Godley F, III, Meitzen J, Nahman-Averbuch H, O'Neal MA, Yeomans D, Santoro N, Riggins N, Edvinsson L. How sex hormones affect migraine: an interdisciplinary preclinical research panel review. J Pers Med 2024;14:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hassan S, Muere A, Einstein G. Ovarian hormones and chronic pain: a comprehensive review. PAIN 2014;155:2448–60. [DOI] [PubMed] [Google Scholar]
- [32].Hellman KM, Oladosu FA, Garrison EF, Roth GE, Dillane KE, Tu FF. Circulating sex steroids and bladder pain sensitivity in dysmenorrhea. Mol Pain 2021;17:17448069211035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Joura EA, Zeisler H, Bancher-Todesca D, Sator MO, Schneider B, Gitsch G. Short-term effects of topical testosterone in vulvar lichen sclerosus. Obstet Gynecol 1997;89:297–9. [DOI] [PubMed] [Google Scholar]
- [34].Keogh E. Sex and gender differences in pain: past, present, and future. PAIN 2022;163(suppl 1):S108–16. [DOI] [PubMed] [Google Scholar]
- [35].Kerem M, Akbayrak T, Bumin G, Yigiter K, Armutlu K, Kerimoglu D. A correlation between sex hormone levels and pressure pain threshold and tolerance in healthy women. Pain Clinic 2002;14:43–7. [Google Scholar]
- [36].Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. PAIN 2017;158:194–211. [DOI] [PubMed] [Google Scholar]
- [37].Labrie F, Archer DF, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dubé R, Côté I, Labrie C, Lavoie L, Berger L, Gilbert L, Martel C, Balser J. Intravaginal dehydroepiandrosterone (prasterone), a highly efficient treatment of dyspareunia. Climacteric 2011;14:282–8. [DOI] [PubMed] [Google Scholar]
- [38].Labrie F, Derogatis L, Archer DF, Koltun W, Vachon A, Young D, Frenette L, Portman D, Montesino M, Côté I, Parent J, Lavoie L, Beauregard A, Martel C, Vaillancourt M, Balser J, Moyneur É, Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med 2015;12:2401–12. [DOI] [PubMed] [Google Scholar]
- [39].Lesnak JB, Inoue S, Lima L, Rasmussen L, Sluka KA. Testosterone protects against the development of widespread muscle pain in mice. PAIN 2020;161:2898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Máximo MM, Silva PS, Vieira CS, Gonçalvez TM, Rosa-E-Silva JC, Candido-Dos-Reis FJ, Nogueira AA, Poli-Neto OB. Low-dose progestin-releasing contraceptives are associated with a higher pain threshold in healthy women. Fertil Steril 2015;104:1182–9. [DOI] [PubMed] [Google Scholar]
- [41].McGuirt AF, Brezing CA. Opioid-induced hypogonadism in opioid use disorder, its role in negative reinforcement, and implications for treatment and retention. Am J Drug Alcohol Abuse 2024;50:132–8. [DOI] [PubMed] [Google Scholar]
- [42].Miller WL, Auchus RJ. The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biol 2019;17:e3000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 2012;13:859–66. [DOI] [PubMed] [Google Scholar]
- [44].Naamneh Elzenaty R, du Toit T, Flück CE. Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab 2022;36:101665. [DOI] [PubMed] [Google Scholar]
- [45].Nahman-Averbuch H, Callahan D, Darken R, Haroutounian S. Harnessing the conditioned pain modulation response in migraine diagnosis, outcome prediction, and treatment—a narrative review. Headache 2023;63:1167–77. [DOI] [PubMed] [Google Scholar]
- [46].Nahman-Averbuch H, Granovsky Y, Coghill RC, Yarnitsky D, Sprecher E, Weissman-Fogel I. Waning of “conditioned pain modulation”: a novel expression of subtle pronociception in migraine. Headache 2013;53:1104–15. [DOI] [PubMed] [Google Scholar]
- [47].Nahman-Averbuch H, Nir RR, Sprecher E, Yarnitsky D. Psychological factors and conditioned pain modulation: a meta-analysis. Clin J Pain 2016;32:541–54. [DOI] [PubMed] [Google Scholar]
- [48].Nahman-Averbuch H, Piché M, Bannister K, Coghill RC. Involvement of propriospinal processes in conditioned pain modulation. PAIN 2024;165:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nahman-Averbuch H, Shefi T, Schneider VJ, II, Li D, Ding L, King CD, Coghill RC. Quantitative sensory testing in patients with migraine: a systematic review and meta-analysis. PAIN 2018;159:1202–23. [DOI] [PubMed] [Google Scholar]
- [50].Nahman-Averbuch H, Timmers I. Neural mechanisms underlying the conditioned pain modulation response: a narrative review of neuroimaging studies. PAIN 2023;164:e25–46. [DOI] [PubMed] [Google Scholar]
- [51].O'Rourke TK, Jr, Wosnitzer MS. Opioid-induced androgen deficiency (OPIAD): diagnosis, management, and literature review. Curr Urol Rep 2016;17:76. [DOI] [PubMed] [Google Scholar]
- [52].Okifuji A, Turk DC. Sex hormones and pain in regularly menstruating women with fibromyalgia syndrome. J Pain 2006;7:851–9. [DOI] [PubMed] [Google Scholar]
- [53].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pan LH, Chen SP, Ling YH, Wang YF, Lai KL, Liu HY, Chen WT, Huang WJ, Coppola G, Treede RD, Wang SJ. Salivary testosterone levels and pain perception exhibit sex-specific association in healthy adults but not in patients with migraine. J Pain 2024;25:104575. [DOI] [PubMed] [Google Scholar]
- [55].Patacchioli FR, Monnazzi P, Simeoni S, De Filippis S, Salvatori E, Coloprisco G, Martelletti P. Salivary cortisol, dehydroepiandrosterone-sulphate (DHEA-S) and testosterone in women with chronic migraine. J Headache Pain 2006;7:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pogatzki-Zahn EM, Drescher C, Englbrecht JS, Klein T, Magerl W, Zahn PK. Progesterone relates to enhanced incisional acute pain and pinprick hyperalgesia in the luteal phase of female volunteers. PAIN 2019;160:1781–93. [DOI] [PubMed] [Google Scholar]
- [57].Poli-Neto O, Silva P, Gonçalvez TM, Máximo M, Rosa-e-Silva J, Candido-dos-Reis F, Nogueira A. Hormone therapy is not associated to pain thresholds in healthy postmenopausal women. Clin Exp Obstet Gynecol 2019;46:66–71. [Google Scholar]
- [58].Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—part 1: are there really differences between women and men? PAIN 2012;153:602–18. [DOI] [PubMed] [Google Scholar]
- [59].Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and pain perception—part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? PAIN 2012;153:619–35. [DOI] [PubMed] [Google Scholar]
- [60].Razzi S, Luisi S, Calonaci F, Altomare A, Bocchi C, Petraglia F. Efficacy of vaginal danazol treatment in women with recurrent deeply infiltrating endometriosis. Fertil Steril 2007;88:789–94. [DOI] [PubMed] [Google Scholar]
- [61].Rezaii T, Hirschberg AL, Carlstrom K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. J Pain 2012;13:646–55. [DOI] [PubMed] [Google Scholar]
- [62].Rhoden EL, Averbeck MA, Teloken PE. Androgen replacement in men undergoing treatment for prostate cancer. J Sex Med 2008;5:2202–8. [DOI] [PubMed] [Google Scholar]
- [63].Rhudy JL, Bartley EJ, Palit S, Kerr KL, Kuhn BL, Martin SL, Delventura JL, Terry EL. Do sex hormones influence emotional modulation of pain and nociception in healthy women? Biol Psychol 2013;94:534–44. [DOI] [PubMed] [Google Scholar]
- [64].Ribeiro-Dasilva MC, Shinal RM, Glover T, Williams RS, Staud R, Riley JL, III, Fillingim RB. Evaluation of menstrual cycle effects on morphine and pentazocine analgesia. PAIN 2011;152:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 2007;92:405–13. [DOI] [PubMed] [Google Scholar]
- [66].RStudio. RStudio: integrated development environment for R. Boston, MA; 2012. Available at: http://www.rstudio.org/. Accessed 2024. [Google Scholar]
- [67].Schertzinger M, Wesson-Sides K, Parkitny L, Younger J. Daily fluctuations of progesterone and testosterone are associated with fibromyalgia pain severity. J Pain 2018;19:410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav 2009;55:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology 2009;150:3690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Scioli-Salter E, Forman DE, Otis JD, Tun C, Allsup K, Marx CE, Hauger RL, Shipherd JC, Higgins D, Tyzik A, Rasmusson AM. Potential neurobiological benefits of exercise in chronic pain and posttraumatic stress disorder: pilot study. J Rehabil Res Dev 2016;53:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 2005;26:163–74. [DOI] [PubMed] [Google Scholar]
- [72].Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. PAIN 2003;103:285–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Team tRC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: http://wwwR-projectorg/2014. Accessed 2024. [Google Scholar]
- [74].Teepker M, Peters M, Vedder H, Schepelmann K, Lautenbacher S. Menstrual variation in experimental pain: correlation with gonadal hormones. Neuropsychobiology 2010;61:131–40. [DOI] [PubMed] [Google Scholar]
- [75].Tesarz J, Schuster AK, Hartmann M, Gerhardt A, Eich W. Pain perception in athletes compared to normally active controls: a systematic review with meta-analysis. PAIN 2012;153:1253–62. [DOI] [PubMed] [Google Scholar]
- [76].Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- [77].Vincent K, Stagg CJ, Warnaby CE, Moore J, Kennedy S, Tracey I. “Luteal analgesia”: progesterone dissociates pain intensity and unpleasantness by influencing emotion regulation networks. Front Endocrinol (Lausanne) 2018;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Brain imaging reveals that engagement of descending inhibitory pain pathways in healthy women in a low endogenous estradiol state varies with testosterone. PAIN 2013;154:515–24. [DOI] [PubMed] [Google Scholar]
- [79].Vollert J, Trewartha N, Kemkowski D, Cremer AF, Zahn PK, Segelcke D, Pogatzki-Zahn EM. Conditioned pain modulation and offset analgesia: influence of sex, sex hormone levels and menstrual cycle on the magnitude and retest reliability in healthy participants. Eur J Pain 2022;26:1938–49. [DOI] [PubMed] [Google Scholar]
- [80].White HD, Brown LA, Gyurik RJ, Manganiello PD, Robinson TD, Hallock LS, Lewis LD, Yeo KT. Treatment of pain in fibromyalgia patients with testosterone gel: pharmacokinetics and clinical response. Int Immunopharmacol 2015;27:249–56. [DOI] [PubMed] [Google Scholar]
- [81].Yamamotova A, Kmoch V, Papezova H. Role of dehydroepiandrosterone and cortisol in nociceptive sensitivity to thermal pain in anorexia nervosa and healthy women. Neuro Endocrinol Lett 2012;33:401–5. [PubMed] [Google Scholar]
- [82].Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–5. [DOI] [PubMed] [Google Scholar]
- [83].Yassin A, AlRumaihi K, Alzubaidi R, Alkadhi S, Al Ansari A. Testosterone, testosterone therapy and prostate cancer. Aging Male 2019;22:219–27. [DOI] [PubMed] [Google Scholar]
- [84].Zhang L, Zhao Y, Liu X, Chen J, Sun M, Zhang J, Zhang W. Changes in sex hormones and their interactions are related to pain perception between different menstrual subphases. Am J Physiol Regul Integr Comp Physiol 2023;325:R280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhuo S, Zhang Y, Lin C, Peng W. Testosterone administration enhances the expectation and perception of painful and non-painful somatosensory stimuli. Psychoneuroendocrinology 2023;152:106081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A296.