Abstract
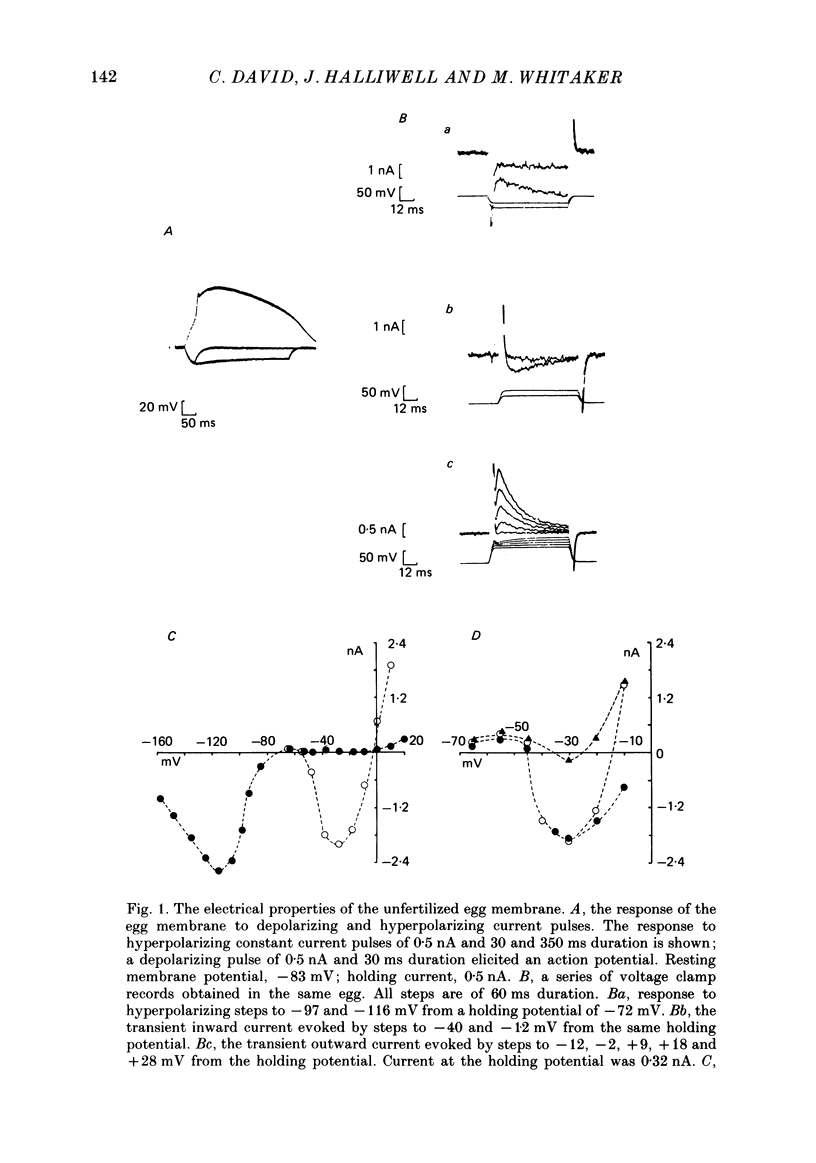
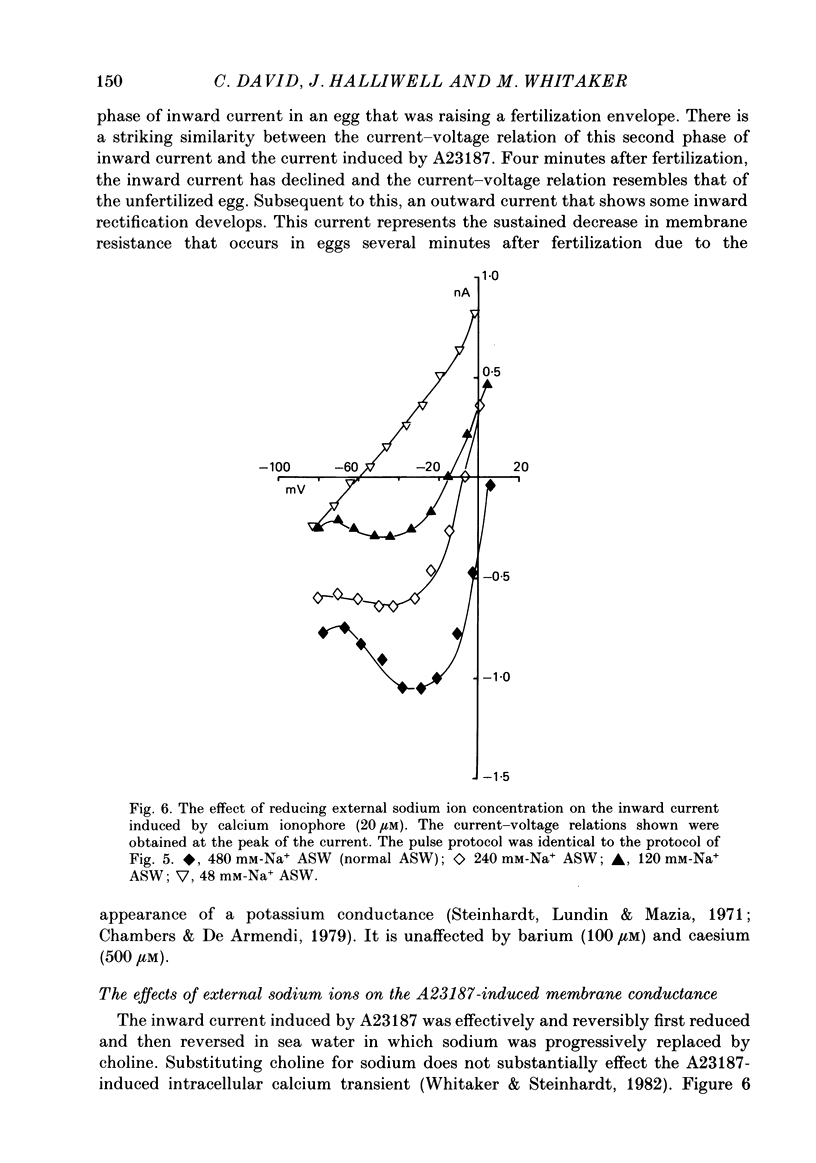
1. The ionic currents that underly the fertilization potential of sea urchin eggs were studied in Lytechinus pictus using a single-electrode voltage clamp technique. 2. In unfertilized eggs, a transient inward current was activated at membrane potentials more positive than -45 mV. The maximum amplitude of the current was 0.56 +/- 0.35 nA (mean +/- S.D., n = 33) at a membrane potential of -35 to -25 mV. 3. The amplitude of this transient inward current was decreased by reducing the external concentration of calcium ions and by substituting barium or strontium ions for calcium in the external medium. Cobalt (10-20 mM) and gadolinium (200-500 microM) ions reduced the amplitude of this current in the presence of calcium ions. 4. A transient outward current was activated in unfertilized eggs at membrane potentials more positive than -10 mV. This current inactivates with a time constant of 16 ms at a membrane potential of -9 mV and re-activates over a period of several seconds at a membrane potential of -72 mV. 5. When unfertilized eggs were treated with the calcium ionophore A23187 under voltage clamp conditions, an inward current developed. It reached a maximum 30 s after its onset and declined thereafter. By 90 s it had become constant at 10% of its peak value. 6. The inward current induced by A23187 was voltage dependent. It was maximal at -25 mV in the steady state. 7. When eggs were fertilized under voltage clamp conditions, the fertilization current, If, was recorded. At a holding potential of -50 or -70 mV If had the following characteristics: (a) an initial inward shoulder with a duration ranging from 12 to 30 s; (b) an inward current peak that was attained between 40 and 100 s after the onset of the shoulder current and declined over the next 60 s; (c) an outward current that appeared after the inward current had declined. 8. Current-voltage relations obtained during If showed that the late component of the inward current was voltage dependent. It was maximal at -25 mV in the steady state and resembled the late component of the inward current recorded in A23187-activated eggs. 9. These results indicate that the form of the action potential in unfertilized eggs is due to the activation of a transient inward current and an inactivating outward current. The sustained depolarization after fertilization is due to the activation of a voltage-dependent inward current by the increase in intracellular free calcium concentration that occurs at fertilization.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne G. W., Trifaró J. M. The gadolinium ion: a potent blocker of calcium channels and catecholamine release from cultured chromaffin cells. Neuroscience. 1982 Jul;7(7):1615–1622. doi: 10.1016/0306-4522(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., Pressman B. C., Rose B. The activation of sea urchin eggs by the divalent ionophores A23187 and X-537A. Biochem Biophys Res Commun. 1974 Sep 9;60(1):126–132. doi: 10.1016/0006-291x(74)90181-8. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., de Armendi J. Membrane potential, action potential and activation potential of eggs of the sea urchin, Lytechinus variegatus. Exp Cell Res. 1979 Aug;122(1):203–218. doi: 10.1016/0014-4827(79)90575-5. [DOI] [PubMed] [Google Scholar]
- Clapper D. L., Lee H. C. Inositol trisphosphate induces calcium release from nonmitochondrial stores i sea urchin egg homogenates. J Biol Chem. 1985 Nov 15;260(26):13947–13954. [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B., De Belice L. J., Taglietti V. Membrane noise and conductance increase during single spermatozoon-egg interactions. Nature. 1978 Sep 21;275(5677):217–219. doi: 10.1038/275217a0. [DOI] [PubMed] [Google Scholar]
- Docherty R. J. Gadolinium selectively blocks a component of calcium current in rodent neuroblastoma x glioma hybrid (NG108-15) cells. J Physiol. 1988 Apr;398:33–47. doi: 10.1113/jphysiol.1988.sp017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshida S., Yoshii M. Transient and delayed potassium currents in the egg cell membrane of the coelenterate, Renilla koellikeri. J Physiol. 1981 Sep;318:123–141. doi: 10.1113/jphysiol.1981.sp013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976 May 6;261(5555):68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A., Robinson K. R. Membrane potential of the unfertilized sea urchin egg. Dev Biol. 1978 Jan;62(1):215–228. doi: 10.1016/0012-1606(78)90103-3. [DOI] [PubMed] [Google Scholar]
- Lansman J. B. Voltage-clamp study of the conductance activated at fertilization in the starfish egg. J Physiol. 1983 Dec;345:353–372. doi: 10.1113/jphysiol.1983.sp014982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo F. J., Lynn J. W., McCulloh D. H., Chambers E. L. Correlative ultrastructural and electrophysiological studies of sperm-egg interactions of the sea urchin, Lytechinus variegatus. Dev Biol. 1986 Nov;118(1):155–166. doi: 10.1016/0012-1606(86)90083-7. [DOI] [PubMed] [Google Scholar]
- Lynn J. W., Chambers E. L. Voltage clamp studies of fertilization in sea urchin eggs. I. Effect of clamped membrane potential on sperm entry, activation, and development. Dev Biol. 1984 Mar;102(1):98–109. doi: 10.1016/0012-1606(84)90178-7. [DOI] [PubMed] [Google Scholar]
- McCulloh D. H., Lynn J. W., Chambers E. L. Membrane depolarization facilitates sperm entry, large fertilization cone formation, and prolonged current responses in sea urchin oocytes. Dev Biol. 1987 Nov;124(1):177–190. doi: 10.1016/0012-1606(87)90470-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Action potential and non-linear current-voltage relation in starfish oocytes. J Physiol. 1975 Mar;246(1):37–54. doi: 10.1113/jphysiol.1975.sp010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Potassium rectifications of the starfish oocyte membrane and their changes during oocyte maturation. J Physiol. 1975 Mar;246(1):55–78. doi: 10.1113/jphysiol.1975.sp010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K. Electrical excitability in the egg cell membrane of the tunicate. J Physiol. 1974 Apr;238(1):37–54. doi: 10.1113/jphysiol.1974.sp010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J., Bosma M. M. Hormone-induced loss of surface membrane during maturation of starfish oocytes: differential effects on potassium and calcium channels. Dev Biol. 1985 Dec;112(2):396–404. doi: 10.1016/0012-1606(85)90412-9. [DOI] [PubMed] [Google Scholar]
- Moody W. J., Lansman J. B. Developmental regulation of Ca2+ and K+ currents during hormone-induced maturation of starfish oocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):3096–3100. doi: 10.1073/pnas.80.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J. The development of calcium and potassium currents during oogenesis in the starfish, Leptasterias hexactis. Dev Biol. 1985 Dec;112(2):405–413. doi: 10.1016/0012-1606(85)90413-0. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R., Grey R. D. Controversy over the fast, partial, temporary block to polyspermy in sea urchins: a reevaluation. Dev Biol. 1984 May;103(1):1–17. doi: 10.1016/0012-1606(84)90002-2. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977 May;267(2):465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Intracellular pH controls the development of new potassium conductance after fertilization of the sea urchin egg. Exp Cell Res. 1980 Jan;125(1):55–61. doi: 10.1016/0014-4827(80)90188-3. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Time and voltage windows for reversing the electrical block to fertilization. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1436–1439. doi: 10.1073/pnas.81.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle. J Physiol. 1979 Sep;294:497–520. doi: 10.1113/jphysiol.1979.sp012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A. 1974 May;71(5):1915–1919. doi: 10.1073/pnas.71.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Lundin L., Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R., Zucker R., Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977 Jul 1;58(1):185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K., Whitaker M. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol. 1986 Dec;103(6 Pt 1):2333–2342. doi: 10.1083/jcb.103.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]


