Abstract
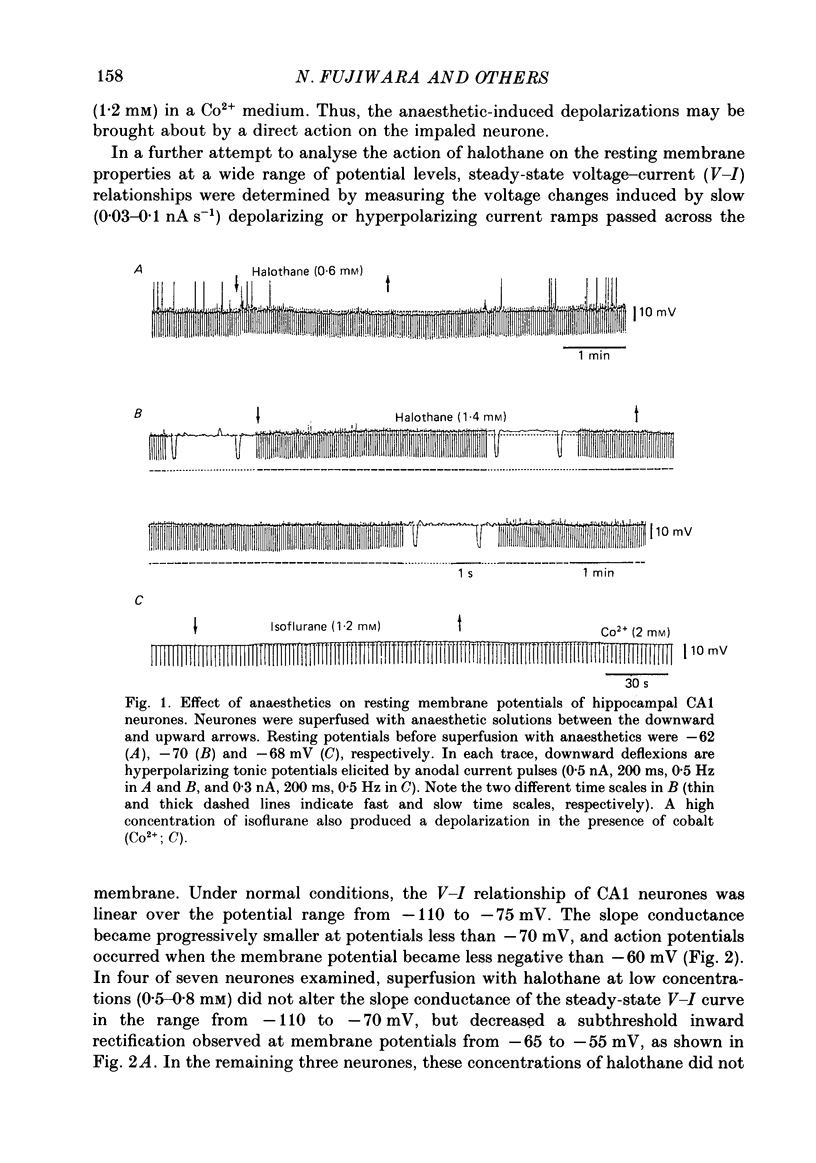
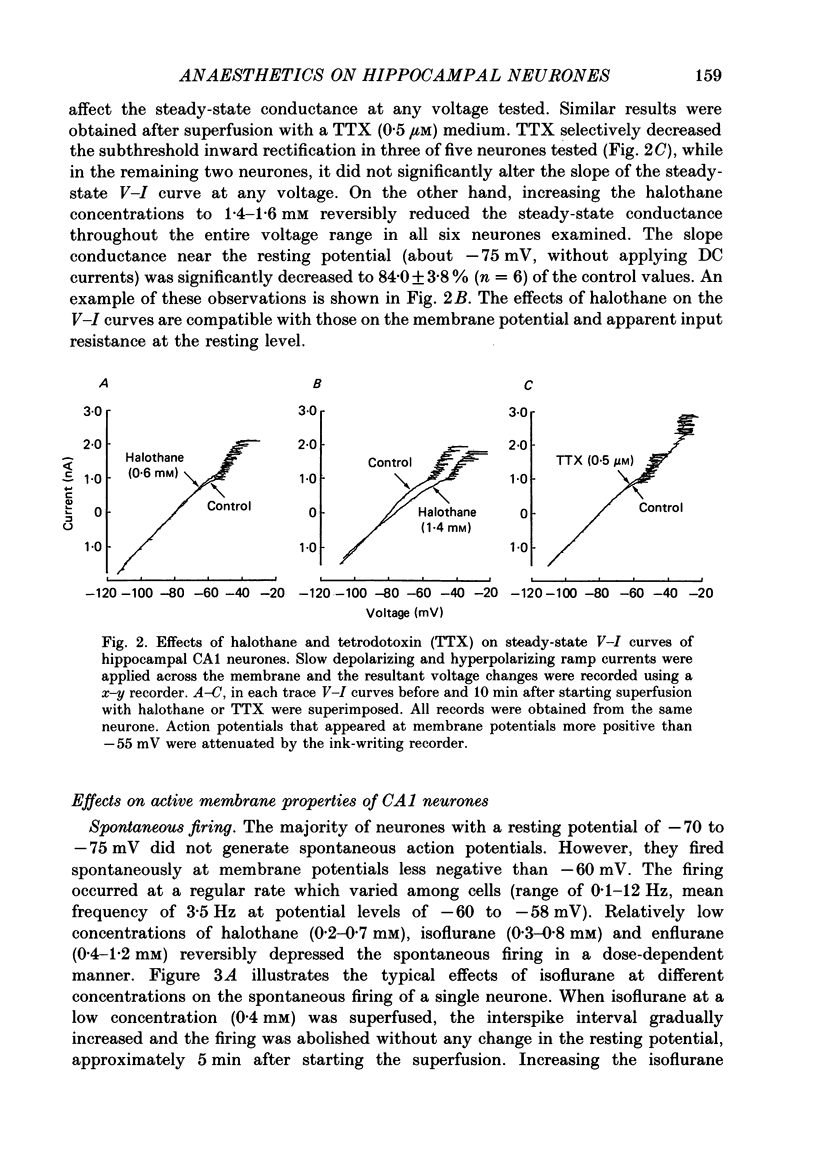
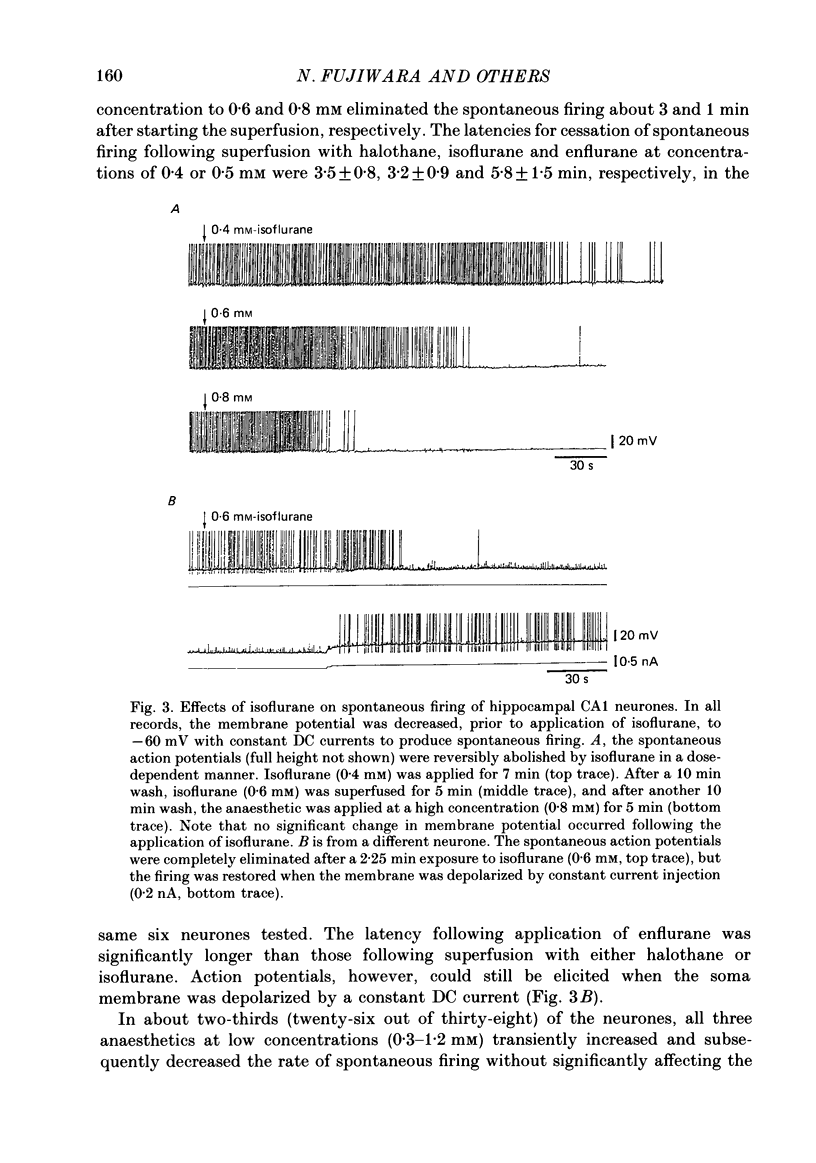
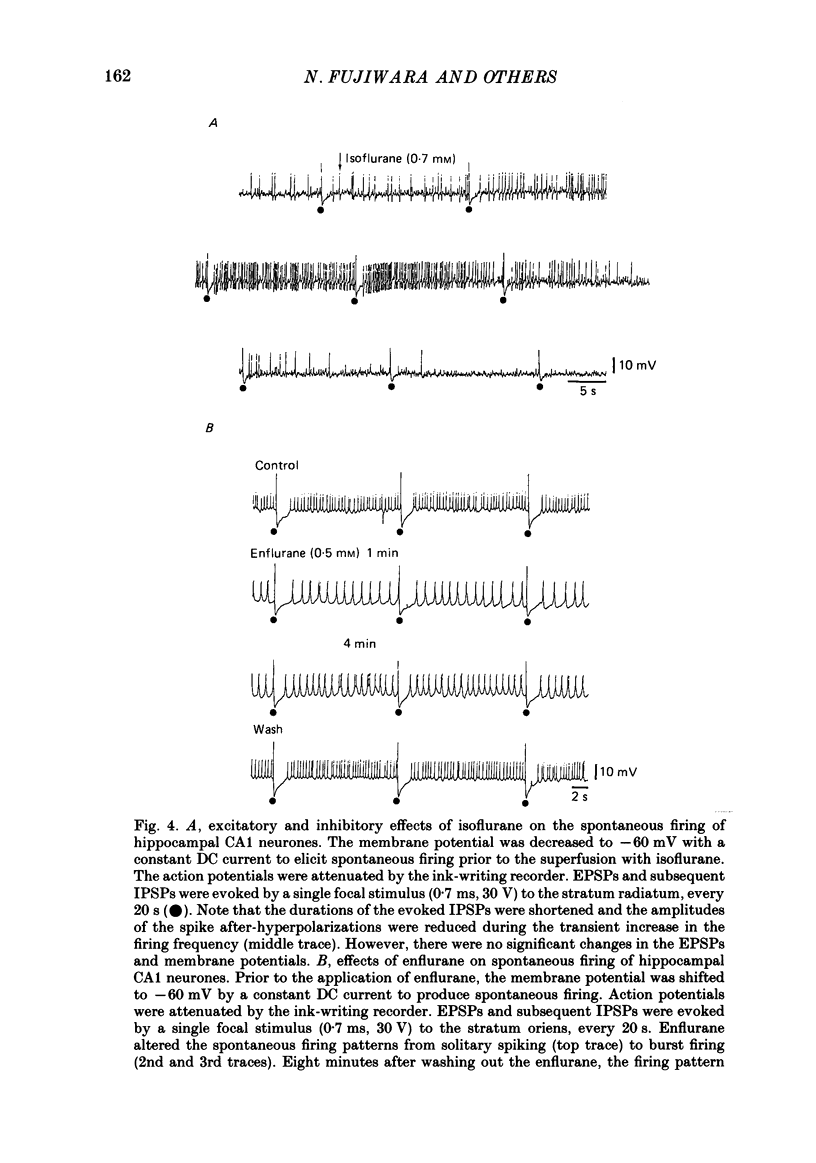
1. The effects of the volatile anaesthetics, halothane, isoflurane and enflurane, on rat hippocampal CA1 and CA3 neurones in in vitro preparations were studied by intracellular recording methods. 2. The three anaesthetics, at concentrations similar to those used clinically (0.2-1.2 mM), initially increased and then subsequently decreased the spontaneous firing of CA1 neurones without affecting the resting membrane properties or the EPSPs evoked by focal stimuli. 3. The anaesthetics at these concentrations depressed both the fast after-hyperpolarization of the soma spike and the post-tetanic hyperpolarization induced by repetitive stimulation. They also decreased the IPSPs evoked by focal stimuli. 4. The threshold for spike generation was gradually elevated by as much as 4-6 mV during application of the anaesthetics at these concentrations. The subthreshold potential oscillations (which are likely to be associated with periodic alterations in non-inactivating Ca2+ and Na+ currents) were enhanced in the low concentrations (0.2-0.5 mM), but were depressed in the high concentrations (0.8-1.2 mM). 5. The results suggest that the transient increase in the firing frequency was caused by a depression of both the spike after-hyperpolarization and the post-tetanic hyperpolarization, and that the reduction of spontaneous firing was mainly due to an elevated threshold for spike generation. 6. The three anaesthetics altered the pattern of spontaneous spike-firing in CA3 neurones from solitary spiking to burst firing without affecting the resting membrane properties. 7. The effects of the anaesthetics on the active membrane properties and the postsynaptic potentials in CA3 neurones were similar to the effects in CA1 neurones. 8. In the majority of CA3 neurones, soma spikes elicited by depolarizing current pulses were followed by a Ca2+-dependent after-depolarization, which was in turn followed by a prolonged after-hyperpolarization (post-burst hyperpolarization). The anaesthetics facilitated the after-depolarizing potential, while they depressed the post-burst hyperpolarization. Combination of the two effects would give rise to the highly stereotyped burst (about 1 Hz in frequency) in the presence of the volatile anaesthetics.
Full text
PDF



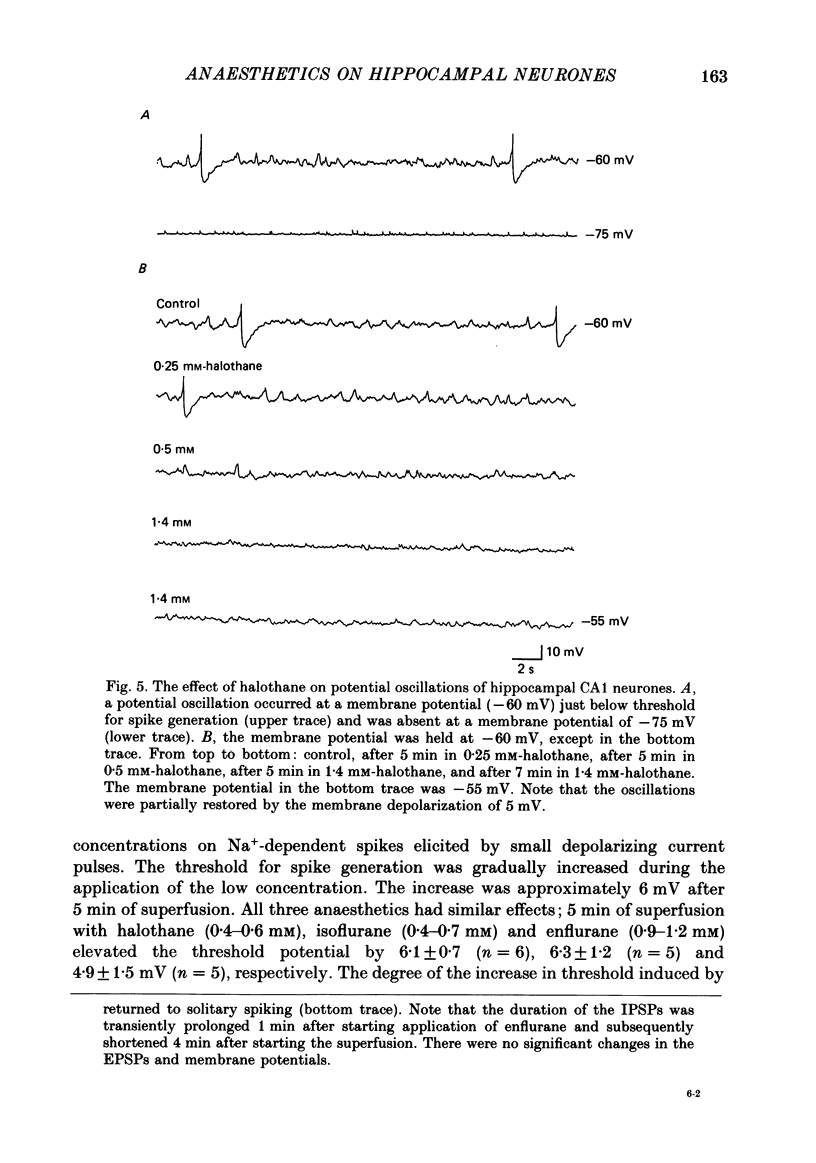

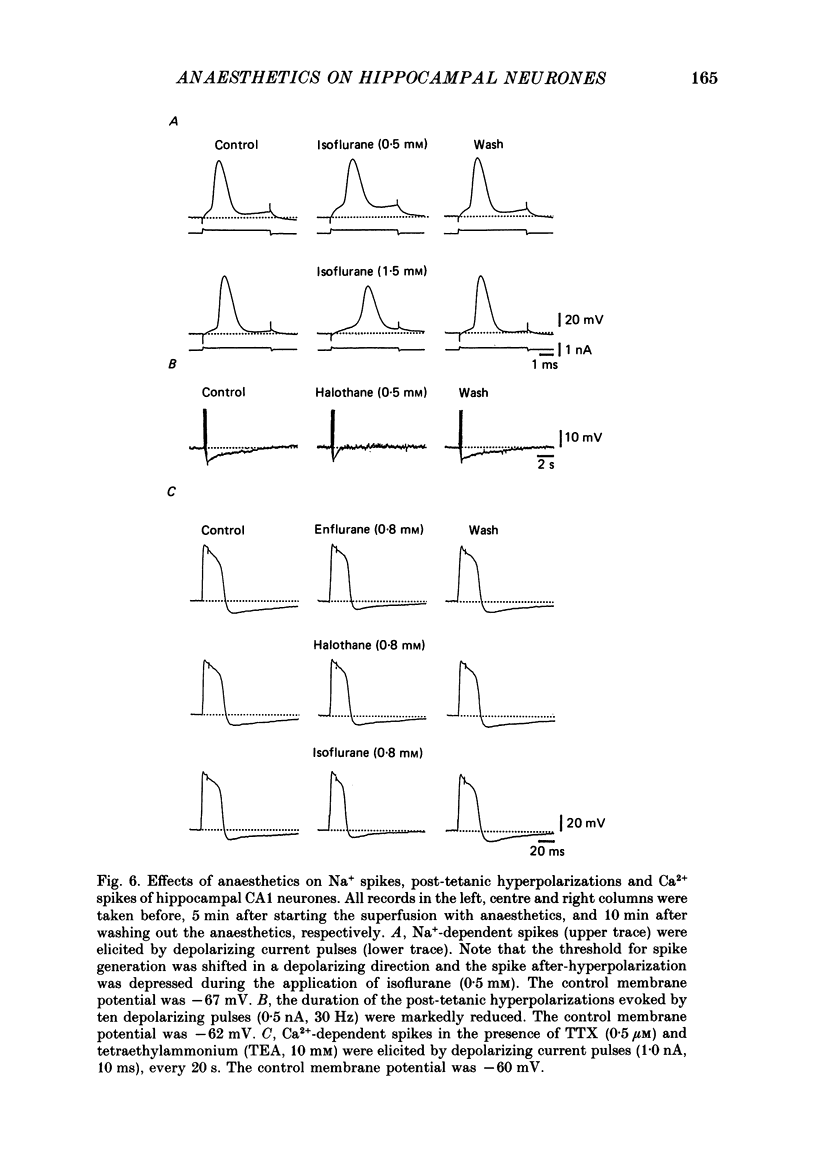
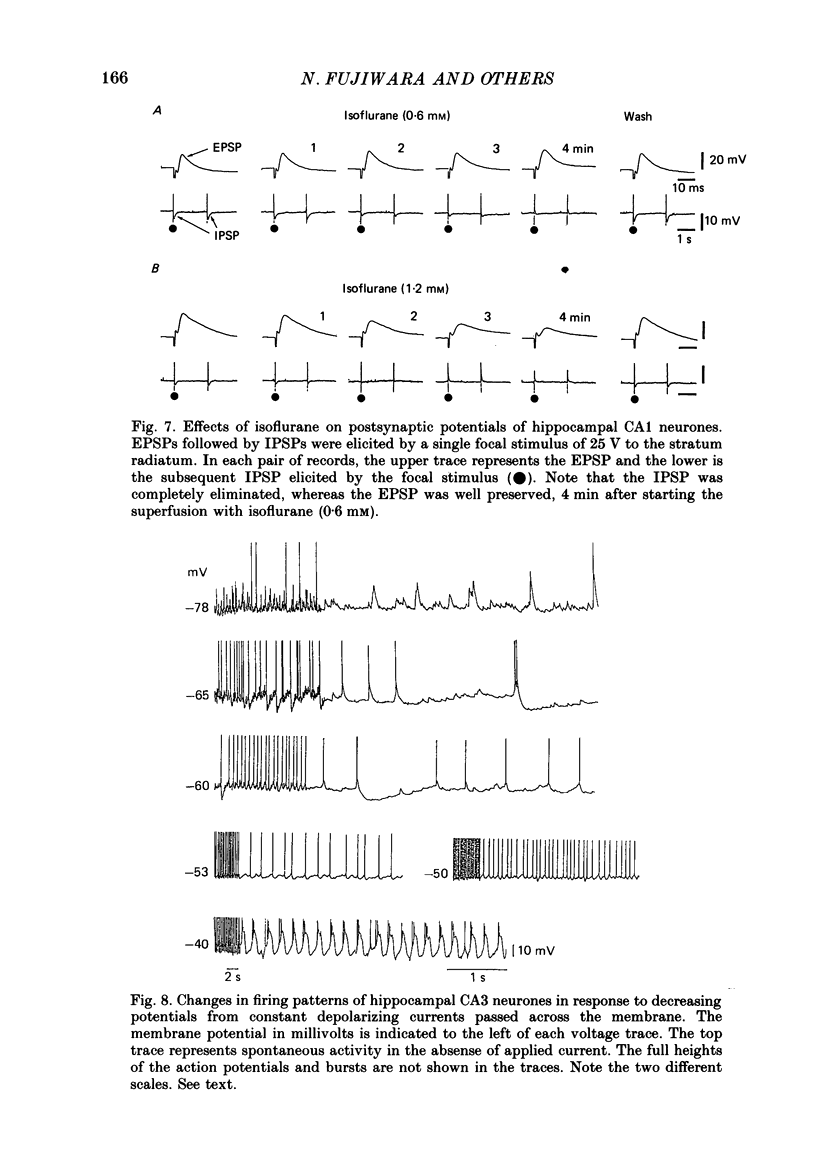
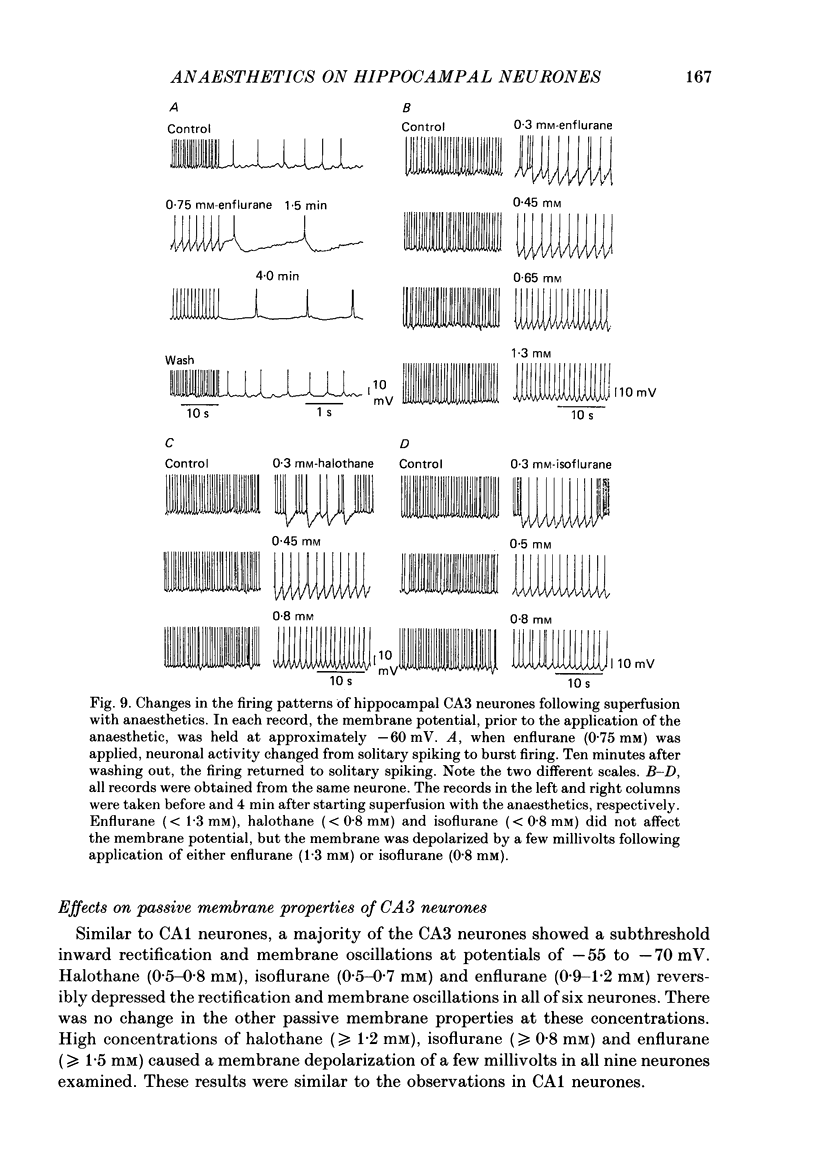







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. A., Griffith W. H. Persistent slow inward calcium current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:303–320. doi: 10.1113/jphysiol.1983.sp014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle P. J., Haas H. L. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol. 1982 May;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E. I., 2nd Isoflurane: a review. Anesthesiology. 1981 Nov;55(5):559–576. doi: 10.1097/00000542-198111000-00014. [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Higashi H., Shimoji K., Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol. 1987 Mar;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J. J., Johnston D. Endogenous nature of spontaneous bursting in hippocampal pyramidal neurons. Cell Mol Neurobiol. 1981 Dec;1(4):325–334. doi: 10.1007/BF00716267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J. J. Picrotoxin-induced epileptiform activity in hippocampus: role of endogenous versus synaptic factors. J Neurophysiol. 1984 May;51(5):1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A., Schwartzkroin P. A. Anomalous inward rectification in hippocampal neurons. J Neurophysiol. 1979 May;42(3):889–895. doi: 10.1152/jn.1979.42.3.889. [DOI] [PubMed] [Google Scholar]
- Johnston D., Hablitz J. J., Wilson W. A. Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature. 1980 Jul 24;286(5771):391–393. doi: 10.1038/286391a0. [DOI] [PubMed] [Google Scholar]
- MacIver M. B., Roth S. H. Enflurane-induced burst firing of hippocampal CA 1 neurones. In vitro studies using a brain slice preparation. Br J Anaesth. 1987 Mar;59(3):369–378. doi: 10.1093/bja/59.3.369. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980 Feb 17;184(1):220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987 Jul;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson A., Peterson E., Wåhlin A. EEG-changes during general anaesthesia with enflurane (Efrane) in comparison with ether. Acta Anaesthesiol Scand. 1978;22(4):339–348. doi: 10.1111/j.1399-6576.1978.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Richards C. D. The action of anaesthetics on synaptic transmission. Gen Pharmacol. 1978;9(5):287–293. doi: 10.1016/0306-3623(78)90063-0. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch. 1980 Feb;383(3):249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Secondary range rhythmic spiking in hippocampal neurons. Brain Res. 1978 Jun 23;149(1):247–250. doi: 10.1016/0006-8993(78)90606-6. [DOI] [PubMed] [Google Scholar]
- Shimoji K., Fujioka H., Fukazawa T., Hashiba M., Maruyama Y. Anesthetics and excitatory/inhibitory responses of midbrain reticular neurons. Anesthesiology. 1984 Aug;61(2):151–155. doi: 10.1097/00000542-198408000-00007. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Amakata Y., Yoshikawa K., Kashimoto T., Izumi F. Catecholamine uptake and release in isolated chromaffin granules exposed to halothane. Anesthesiology. 1980 Nov;53(5):385–389. doi: 10.1097/00000542-198011000-00005. [DOI] [PubMed] [Google Scholar]
- Wade J. G., Stevens W. C. Isoflurane: an anesthetic for the eighties? Anesth Analg. 1981 Sep;60(9):666–682. [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Afterpotential generation in hippocampal pyramidal cells. J Neurophysiol. 1981 Jan;45(1):86–97. doi: 10.1152/jn.1981.45.1.86. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 1978 Dec 29;159(2):385–390. doi: 10.1016/0006-8993(78)90544-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Higashi H., Fujita S., Shimoji K. Selective depression of hippocampal inhibitory postsynaptic potentials and spontaneous firing by volatile anesthetics. Brain Res. 1985 Aug 12;340(2):363–368. doi: 10.1016/0006-8993(85)90933-3. [DOI] [PubMed] [Google Scholar]


