Abstract
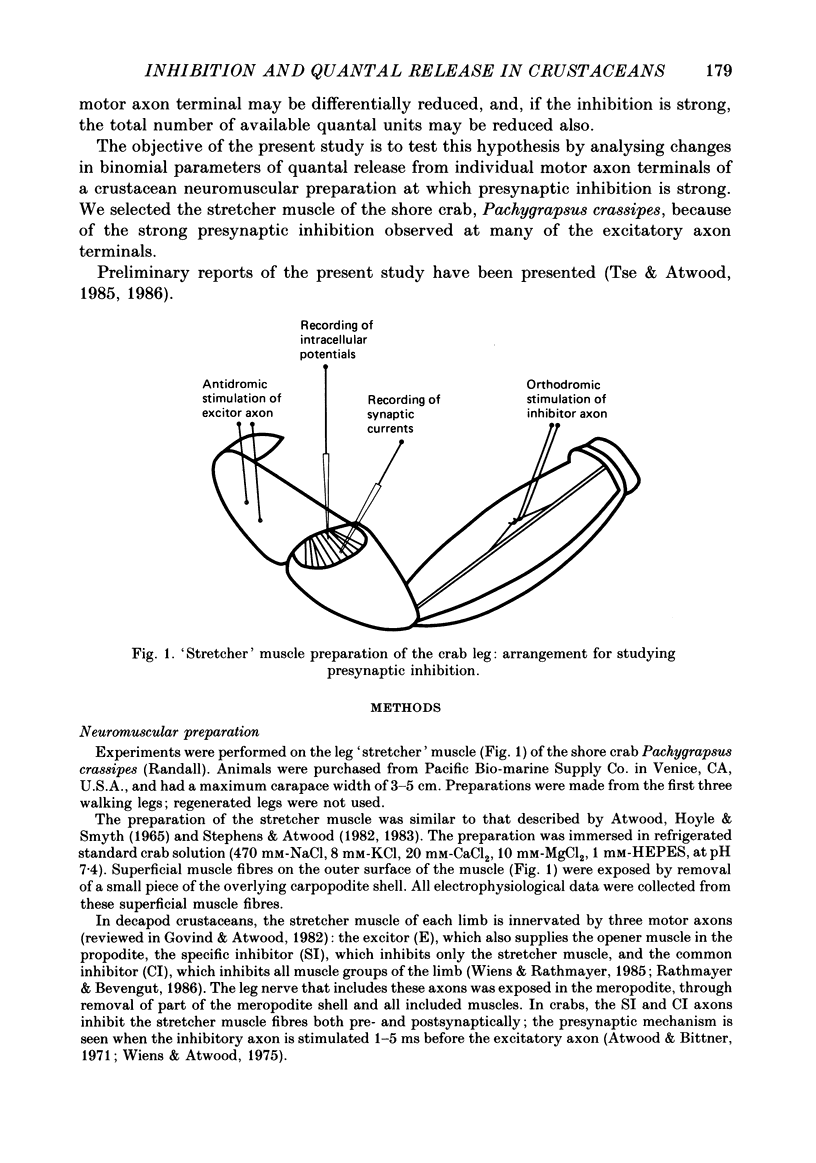
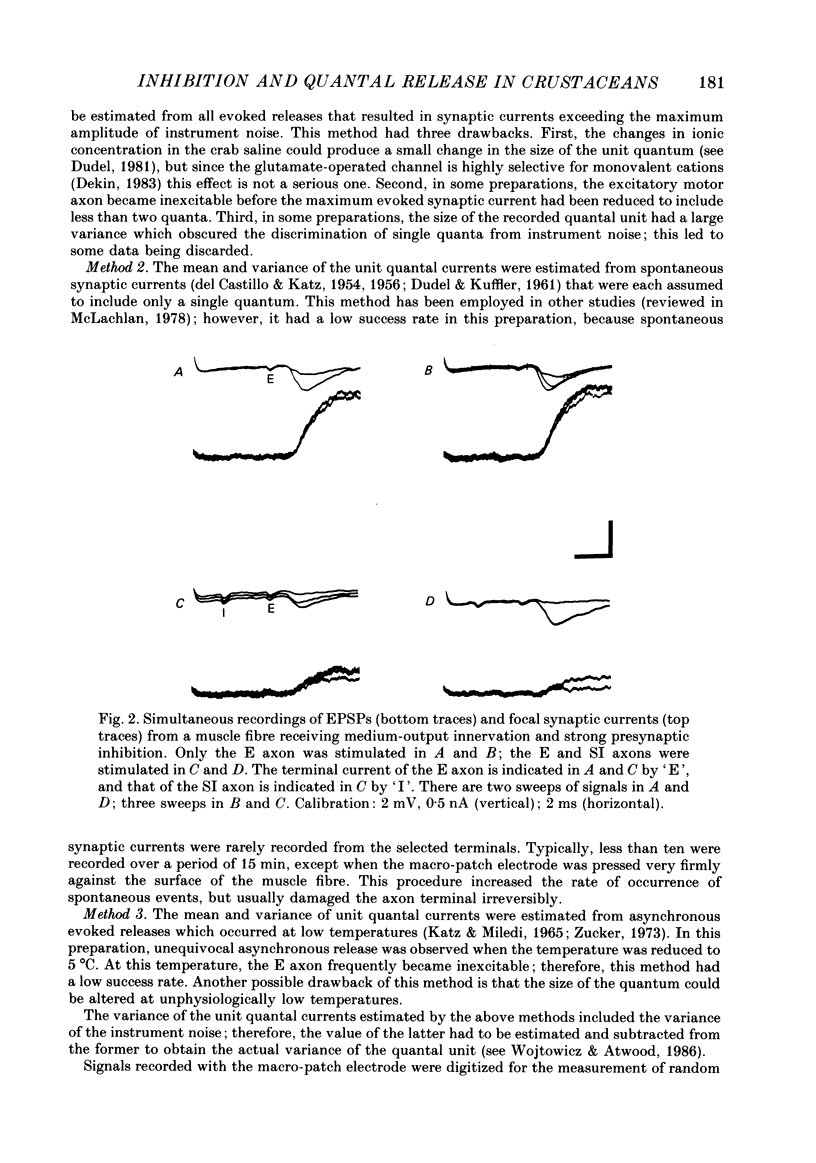
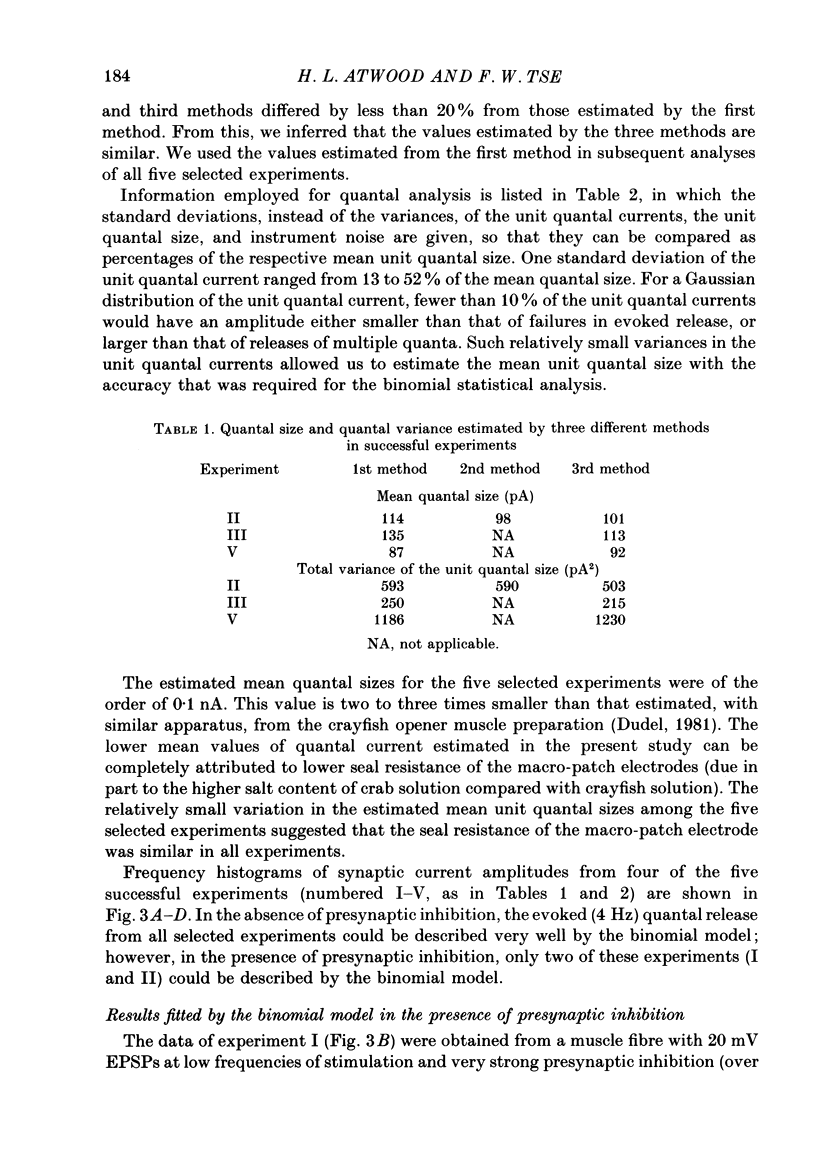
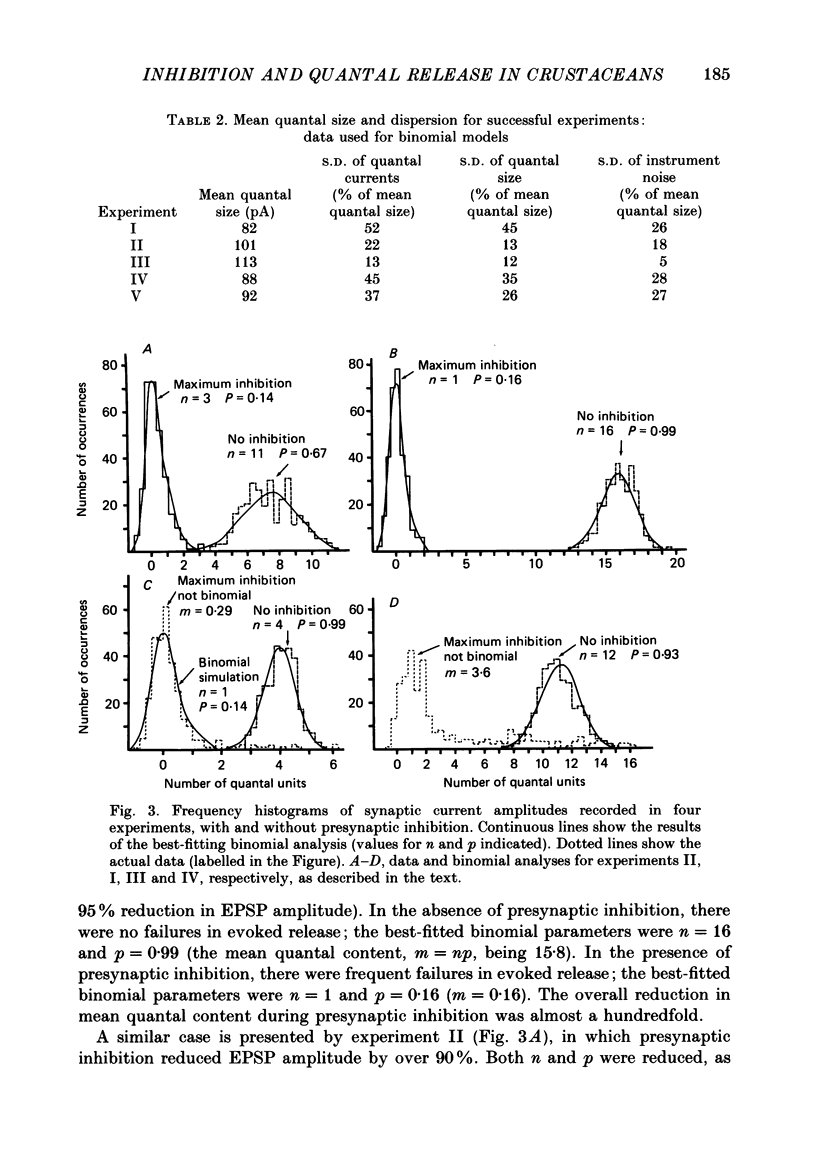
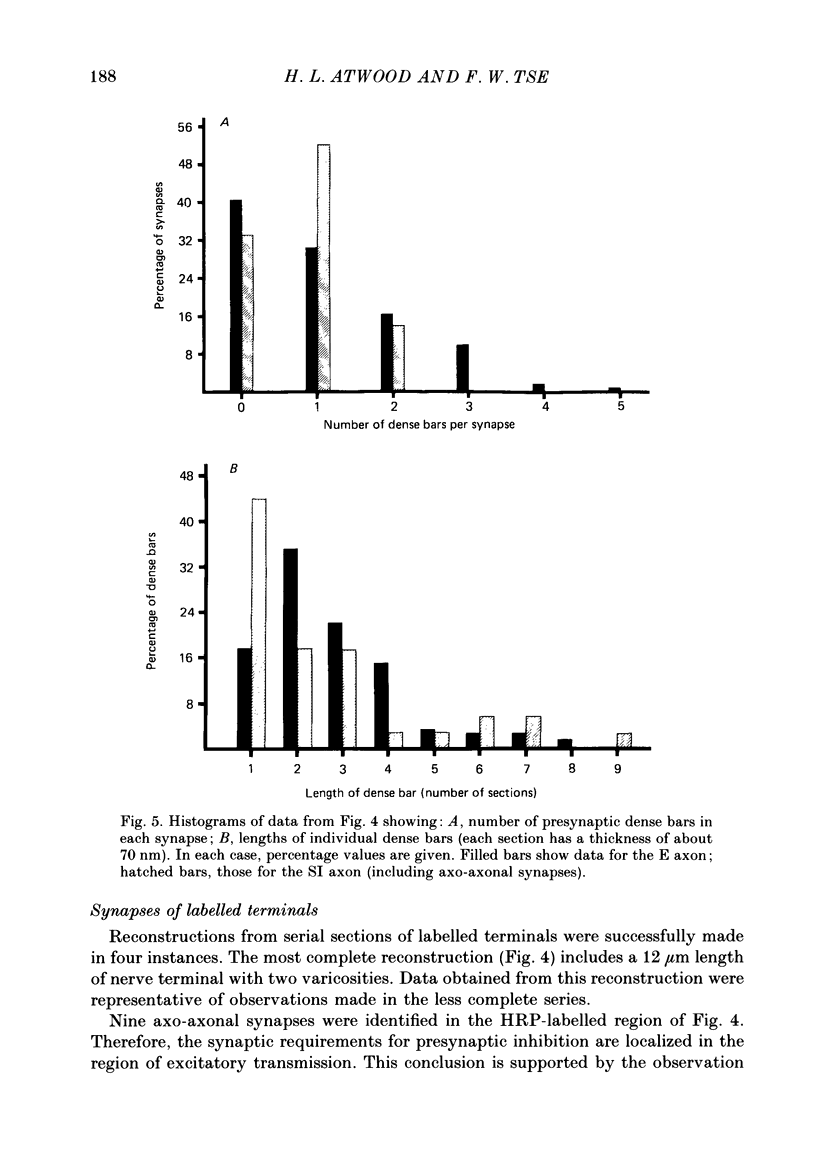
1. The effects of presynaptic inhibition on quantal release of transmitter were investigated at neuromuscular junctions of the motor axon supplying one of the limb muscles of a crab (Pachygrapsus crassipes). 2. Binomial analysis of transmitter release recorded at selected neuromuscular junctions with an extracellular 'macro-patch' electrode indicated high probability of release (p) from a limited number of available sites (n). During presynaptic inhibition, both n and p were reduced. 3. The binomial model provided a good description of results from non-inhibited junctions. During presynaptic inhibition, results from some junctions could be described by the binomial model, while those from other junctions could not. An interpretation of this finding is that presynaptic inhibition differentially affects the probability of release at various release sites of the neuromuscular junctional complex. 4. A morphological study of the region of transmitter release under the macropatch electrode was made. Release-dependent uptake of horseradish peroxidase (HRP) into presynaptic terminals was restricted to the region under the recording electrode, by perfusing the preparation with calcium-free solution containing HRP. Transmitter release, and HRP uptake, occurred only at the site of the electrode, which was filled with a calcium-containing solution. Subsequently, serial sections were prepared for electron microscopy and the region of transmitter release was reconstructed. 5. Numerous axo-axonal synapses were found in the HRP-labelled region. Thus, the morphological prerequisite for presynaptic inhibition exists at the site of transmitter release, and not exclusively at a more remote region. 6. The number of morphologically identified excitatory neuromuscular synapses exceeded the 'release sites' estimated from the binomial model (n) by a wide margin. Morphological differences among synapses were observed. It is proposed that not all morphologically identified synapses participated in transmitter release under the experimental conditions employed. Thus, morphologically defined synapses are likely to be non-uniform in their response properties, including probability of transmitter release (p).
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L., Bittner G. D. Matching of excitatory and inhibitory inputs to crustacean muscle fibers. J Neurophysiol. 1971 Jan;34(1):157–170. doi: 10.1152/jn.1971.34.1.157. [DOI] [PubMed] [Google Scholar]
- Atwood H. L., Govind C. K., Kwan I. Nonhomogeneous excitatory synapses of a crab stomach muscle. J Neurobiol. 1978 Jan;9(1):17–28. doi: 10.1002/neu.480090103. [DOI] [PubMed] [Google Scholar]
- Atwood H. L., Hoyle G., Smyth T., Jr Mechanical and electrical responses of single innervated crab-muscle fibres. J Physiol. 1965 Oct;180(3):449–482. doi: 10.1113/jphysiol.1965.sp007712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L., Kwan I. Synaptic development in the crayfish opener muscle. J Neurobiol. 1976 Jul;7(4):289–312. doi: 10.1002/neu.480070403. [DOI] [PubMed] [Google Scholar]
- Atwood H. L., Stevens J. K., Marin L. Axoaxonal synapse location and consequences for presynaptic inhibition in crustacean motor axon terminals. J Comp Neurol. 1984 May 1;225(1):64–74. doi: 10.1002/cne.902250108. [DOI] [PubMed] [Google Scholar]
- Atwood H. L., Wojtowicz J. M. Short-term and long-term plasticity and physiological differentiation of crustacean motor synapses. Int Rev Neurobiol. 1986;28:275–362. doi: 10.1016/s0074-7742(08)60111-7. [DOI] [PubMed] [Google Scholar]
- Barton S. B., Cohen I. S. Are transmitter release statistics meaningful? Nature. 1977 Jul 21;268(5617):267–268. doi: 10.1038/268267a0. [DOI] [PubMed] [Google Scholar]
- Baxter D. A., Bittner G. D. Intracellular recordings from crustacean motor axons during presynaptic inhibition. Brain Res. 1981 Nov 2;223(2):422–428. doi: 10.1016/0006-8993(81)91159-8. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Perkel D. H., Feldman M. W. Evoked neurotransmitter release: statistical effects of nonuniformity and nonstationarity. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2913–2917. doi: 10.1073/pnas.73.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Forsythe I. D., Redman S. J. Presynaptic inhibition of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia axons. J Physiol. 1987 Feb;383:153–169. doi: 10.1113/jphysiol.1987.sp016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekin M. S. Permeability changes induced by L-glutamate at the crayfish neuromuscular junction. J Physiol. 1983 Aug;341:105–125. doi: 10.1113/jphysiol.1983.sp014795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflugers Arch. 1981 Jul;391(1):35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Freeman A. R., Kashner L. A. Stimulation-dependent alterations in peroxidase uptake at lobster neuromuscular junctions. Science. 1971 Aug 20;173(3998):733–736. doi: 10.1126/science.173.3998.733. [DOI] [PubMed] [Google Scholar]
- Jahromi S. S., Atwood H. L. Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. J Cell Biol. 1974 Nov;63(2 Pt 1):599–613. doi: 10.1083/jcb.63.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Korn H., Triller A., Mallet A., Faber D. S. Fluctuating responses at a central synapse: n of binomial fit predicts number of stained presynaptic boutons. Science. 1981 Aug 21;213(4510):898–901. doi: 10.1126/science.6266015. [DOI] [PubMed] [Google Scholar]
- Lustig C., Parnas H., Segel L. A. On the quantal hypothesis of neurotransmitter release: an explanation for the calcium dependence of the binomial parameters. J Theor Biol. 1986 May 21;120(2):205–213. doi: 10.1016/s0022-5193(86)80174-6. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Presynaptic inhibition: transmitter and ionic mechanisms. Int Rev Neurobiol. 1979;21:217–258. doi: 10.1016/s0074-7742(08)60639-x. [DOI] [PubMed] [Google Scholar]
- Pearce J., Govind C. K., Shivers R. R. Intramembranous organization of lobster excitatory neuromuscular synapses. J Neurocytol. 1986 Apr;15(2):241–252. doi: 10.1007/BF01611660. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. Amplitude fluctuations in synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:135–145. doi: 10.1113/jphysiol.1983.sp014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S., Walmsley B. The time course of synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:117–133. doi: 10.1113/jphysiol.1983.sp014884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Ultrastructural specificity of synaptic sites in nerve terminals mediating both presynaptic and postsynaptic inhibition. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):839–849. doi: 10.1002/cne.901820507. [DOI] [PubMed] [Google Scholar]
- Smith D. O. Variable activation of synaptic release sites at the neuromuscular junction. Exp Neurol. 1983 Jun;80(3):520–528. doi: 10.1016/0014-4886(83)90304-7. [DOI] [PubMed] [Google Scholar]
- Stephens P. J., Atwood H. L. Thermal acclimation in a crustacean neuromuscular system. J Exp Biol. 1982 Jun;98:39–47. doi: 10.1242/jeb.98.1.39. [DOI] [PubMed] [Google Scholar]
- Thompson C. S., Atwood H. L. Synaptic strength and horseradish peroxidase uptake in crayfish nerve terminals. J Neurocytol. 1984 Apr;13(2):267–280. doi: 10.1007/BF01148119. [DOI] [PubMed] [Google Scholar]
- Triller A., Korn H. Transmission at a central inhibitory synapse. III. Ultrastructure of physiologically identified and stained terminals. J Neurophysiol. 1982 Sep;48(3):708–736. doi: 10.1152/jn.1982.48.3.708. [DOI] [PubMed] [Google Scholar]
- Tse F. W., Marin L., Atwood H. L. Focal labeling of axonal terminals with active synapses recorded by an extracellular macro-patch electrode. J Neurosci Methods. 1987 Sep;21(1):17–29. doi: 10.1016/0165-0270(87)90099-9. [DOI] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz J. M., Atwood H. L. Long-term facilitation alters transmitter releasing properties at the crayfish neuromuscular junction. J Neurophysiol. 1986 Mar;55(3):484–498. doi: 10.1152/jn.1986.55.3.484. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]