Abstract
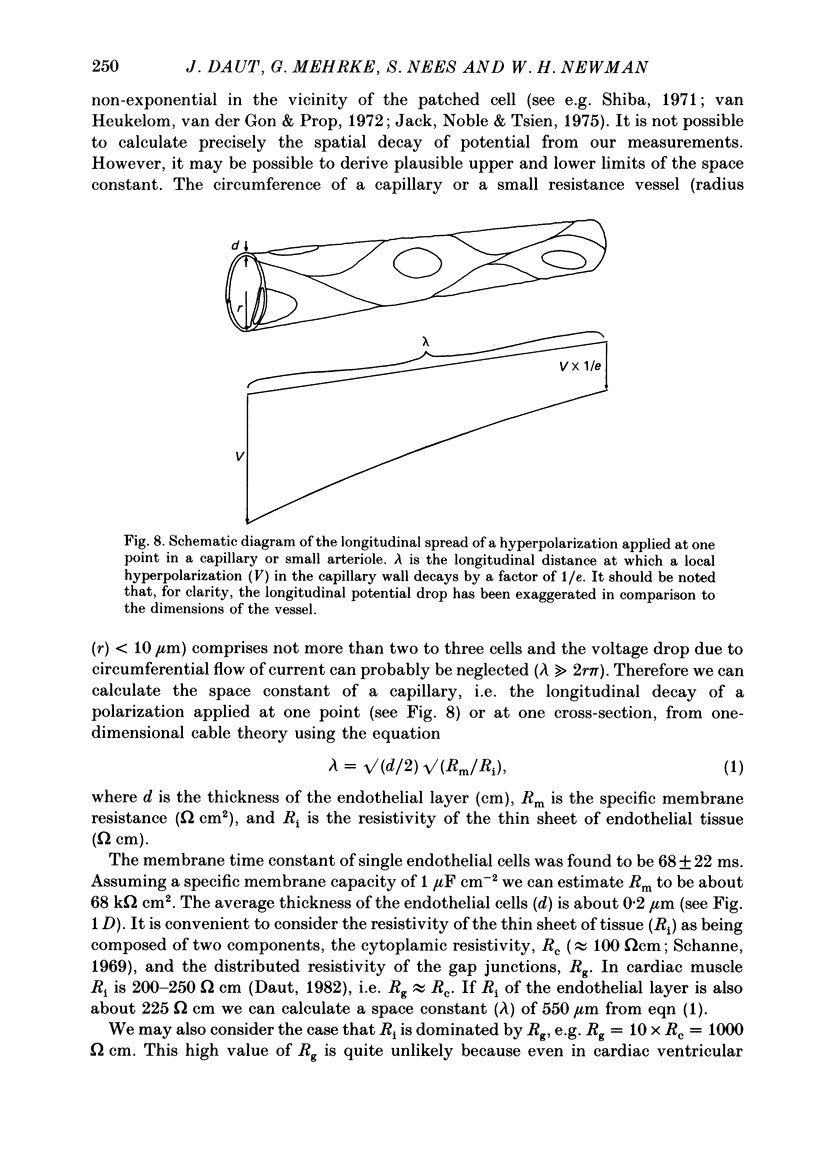
1. Coronary endothelial cells were isolated from adult guinea-pig hearts (Nees, Gerbes & Gerlach, 1981) and the electrical properties of primary cultures were studied using the tight-seal whole-cell recording mode of the patch clamp technique. 2. On the third or fourth day in culture whole-cell clamp records from single coronary endothelial cells were obtained at 37 degrees C. The resting potential was -33 +/- 6 mV (n = 10). The membrane time constant determined with rectangular current pulses was 68 +/- 22 ms (n = 10). 3. In voltage clamp experiments, no time-dependent membrane conductance changes were found in the range -80 to +40 mV. The current-voltage relation was linear in normal physiological salt solution containing 5.4 mM-K+. The input resistance was 1.7 +/- 0.4 G omega. When the external K+ concentration was increased to 116 mM the cells depolarized to about -3 mV and the clamp currents showed marked inward rectification. 4. Between days four and seven in culture the endothelial cells formed confluent monolayers which showed the characteristic 'cobblestone' morphology. The input resistance of cells in a monolayer was 8 +/- 3 M omega, i.e. a factor of 200 lower than that found in single cells. It was concluded that the cells in the confluent monolayer are coupled electrically by gap junctions. 5. Exposure of coronary endothelial cells to K+-free solution for 5 min produced a depolarization of about 8 mV. Upon readmission of normal external K+ the cells transiently hyperpolarized by up to 20 mV. This transient hyperpolarization decayed with a time constant of 1.9 +/- 0.3 min. 6. The transient hyperpolarization could be abolished by application of 2 x 10(-4) M-dihydro-ouabain (DHO). Application of DHO in the steady state produced a depolarization of 8 +/- 1 mV. From these findings it was concluded that coronary endothelial cells possess an electrogenic sodium pump which contributes about -8 mV to the resting potential. 7. From the passive electrical properties of single cells and the morphological data available it was calculated that endothelium in situ may have a large electrical space constant, probably between 250 and 550 microns. 8. The functional implications of the large space constant of the endothelial monolayer are discussed. It is suggested that intra-endothelial conduction of electrical signals from capillaries to the resistance vessels may be involved in the local regulation of blood flow in the intact heart.
Full text
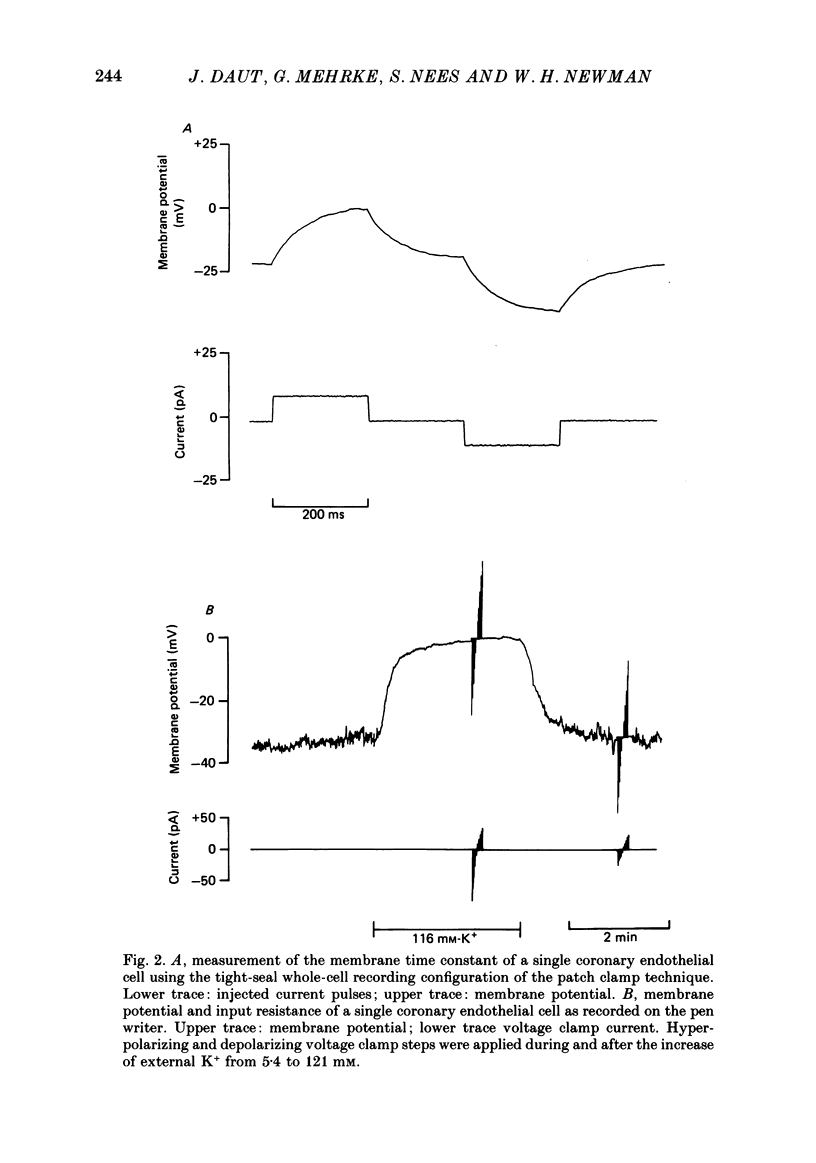
PDF



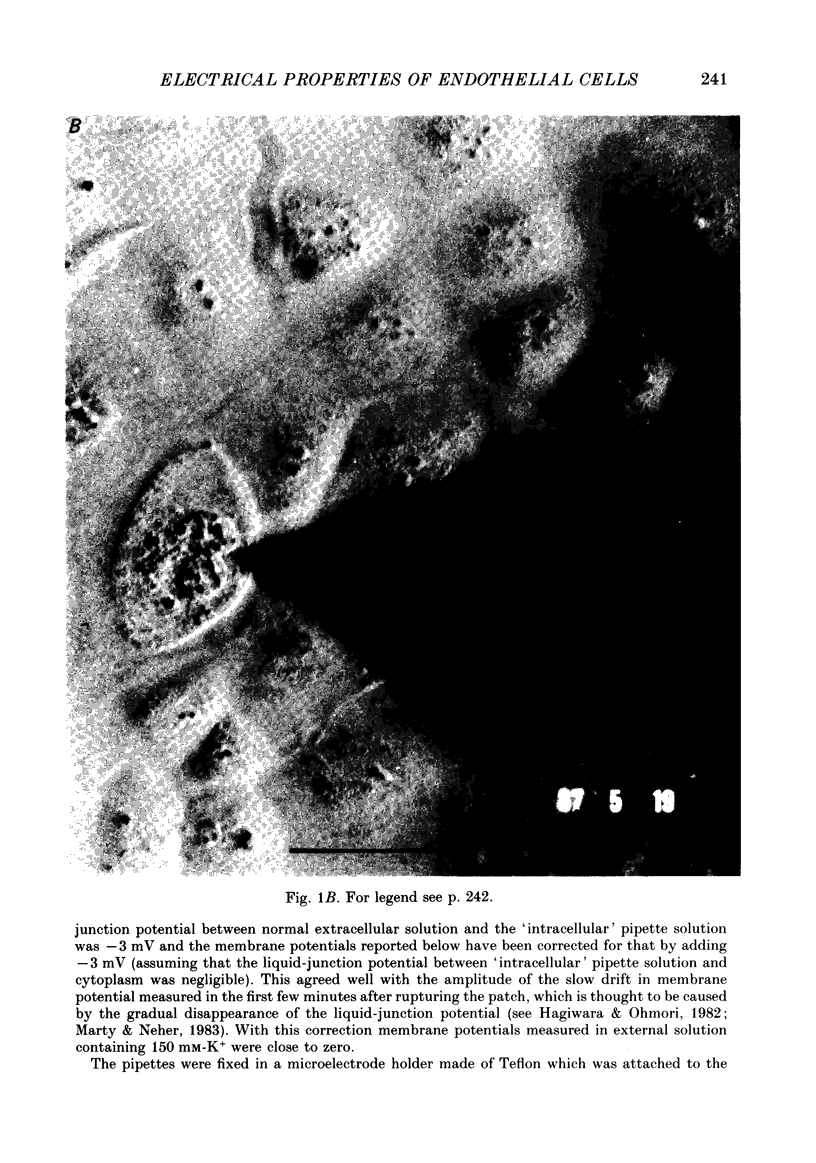


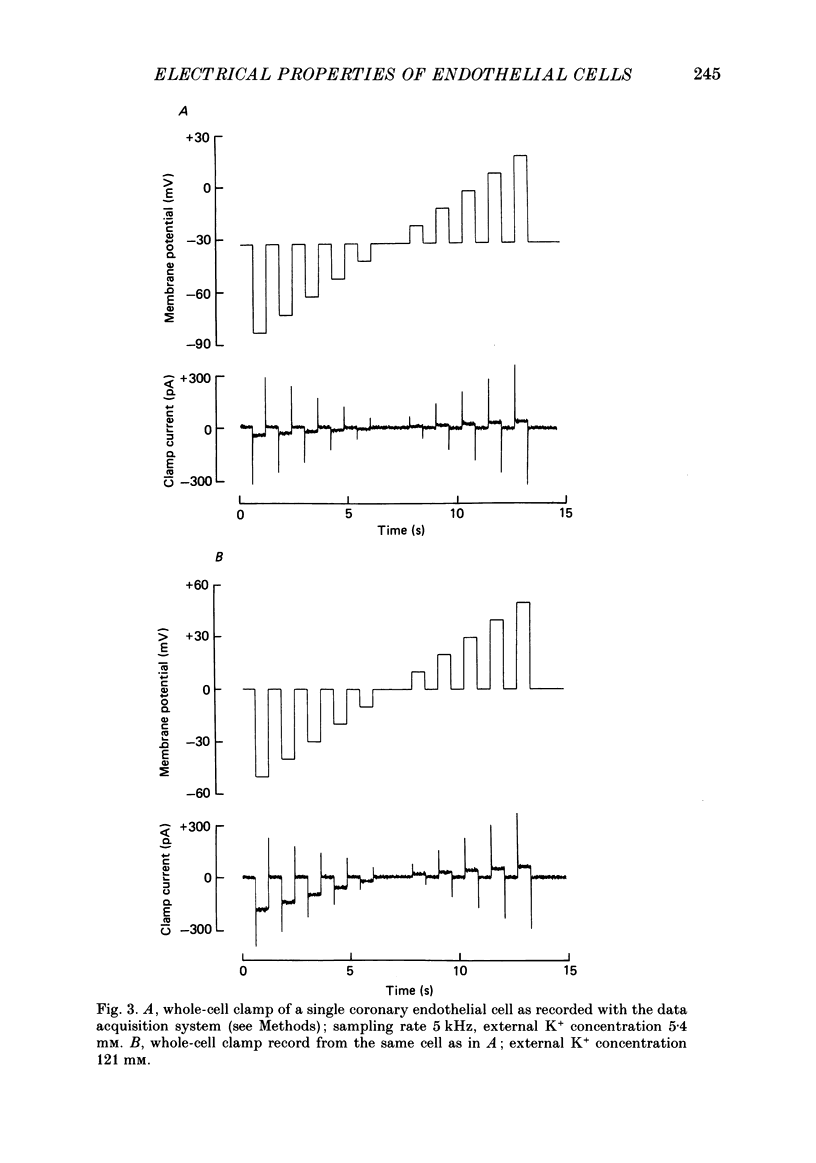
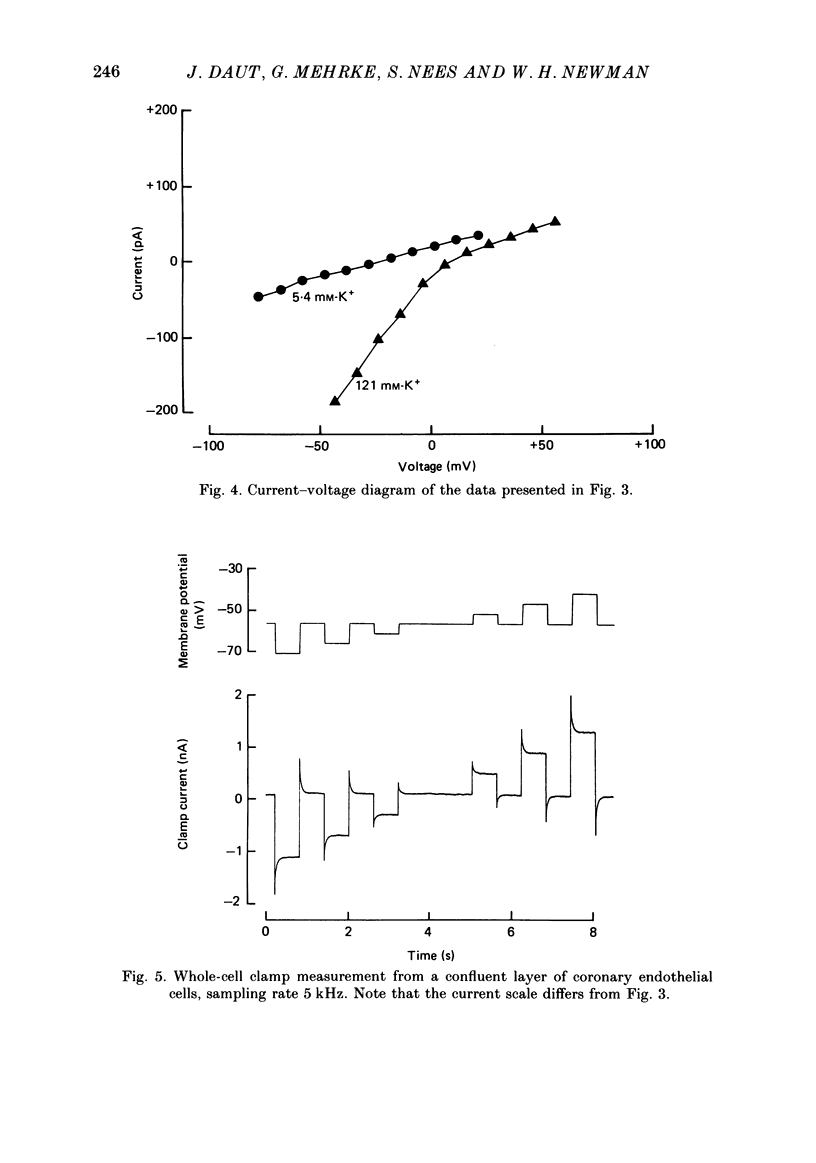

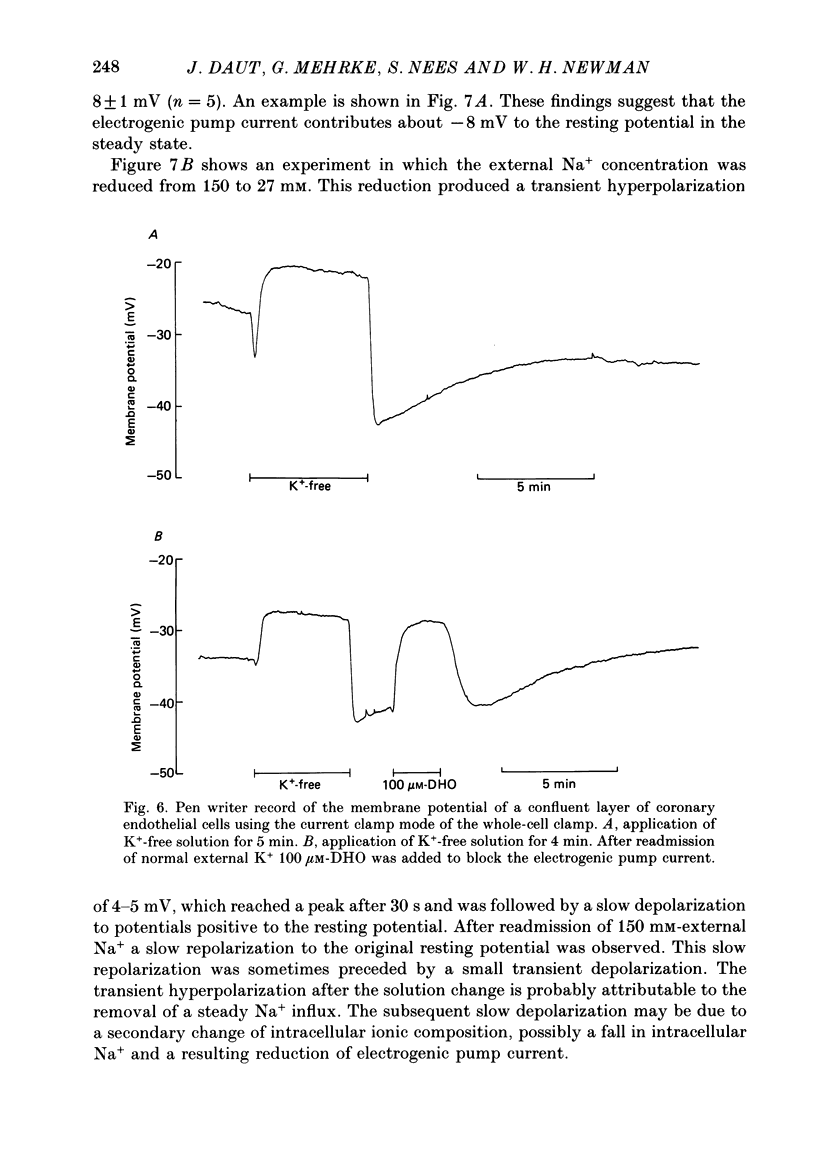




Images in this article
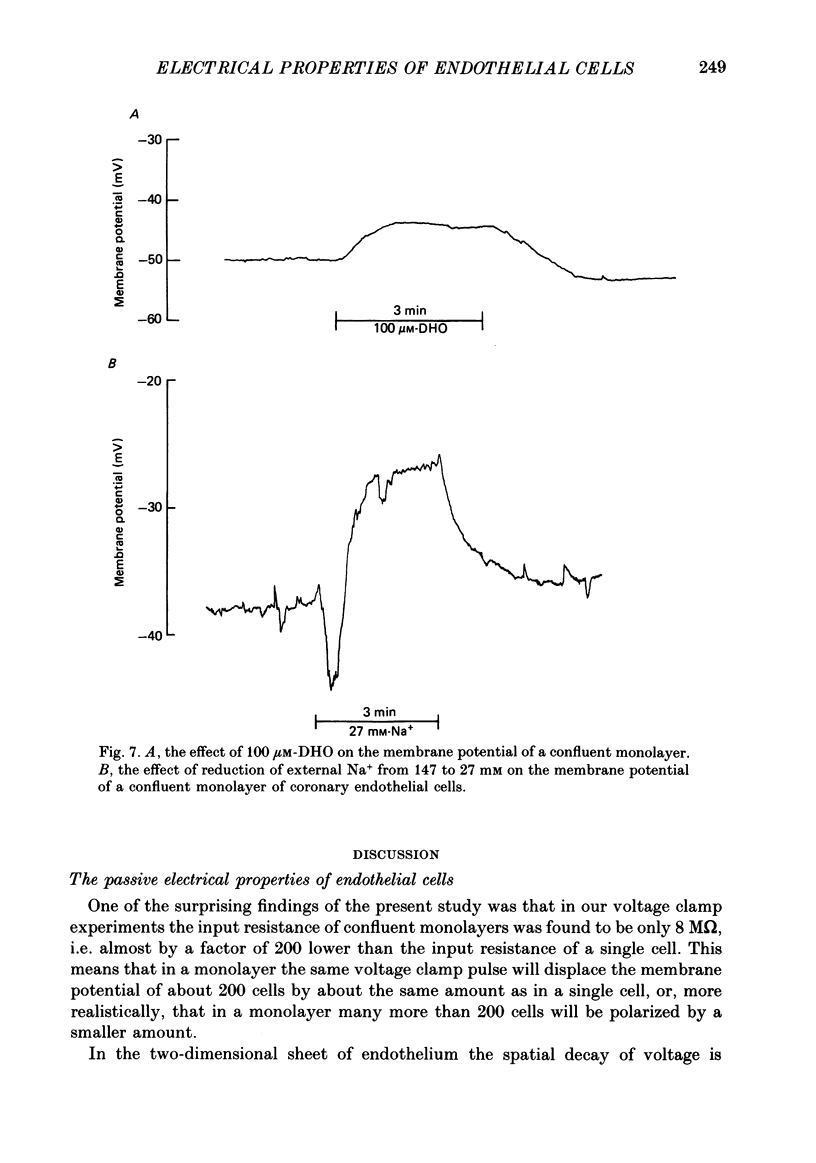
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman K. A., Elijah R. D., Cheeks K. E., Green K. Intracellular potential and pH of rabbit corneal endothelial cells. Curr Eye Res. 1984 Aug;3(8):991–1000. doi: 10.3109/02713688409011745. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Brugnara C., Canessa M., Gimbrone M. A., Jr Bradykinin and vasopressin stimulate Na+-K+-Cl- cotransport in cultured endothelial cells. Am J Physiol. 1986 Jun;250(6 Pt 1):C888–C895. doi: 10.1152/ajpcell.1986.250.6.C888. [DOI] [PubMed] [Google Scholar]
- Cohen I. S., Datyner N. B., Gintant G. A., Mulrine N. K., Pennefather P. Properties of an electrogenic sodium-potassium pump in isolated canine Purkinje myocytes. J Physiol. 1987 Feb;383:251–267. doi: 10.1113/jphysiol.1987.sp016407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Rüdel R. The electrogenic sodium pump in guinea-pig ventricular muscle: inhibition of pump current by cardiac glycosides. J Physiol. 1982 Sep;330:243–264. doi: 10.1113/jphysiol.1982.sp014339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. The passive electrical properties of guinea-pig ventricular muscle as examined with a voltage-clamp technique. J Physiol. 1982 Sep;330:221–242. doi: 10.1113/jphysiol.1982.sp014338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner H., Fröbe U., Busse R., Kohlhardt M. Single nonselective cation channels and Ca2+-activated K+ channels in aortic endothelial cells. J Membr Biol. 1987;98(2):125–133. doi: 10.1007/BF01872125. [DOI] [PubMed] [Google Scholar]
- Freissmuth M., Nees S., Böck M., Schütz W. Binding of [3H]ouabain to endothelial cells derived from various vascular beds. Basic Res Cardiol. 1987 Nov-Dec;82(6):544–550. doi: 10.1007/BF01907224. [DOI] [PubMed] [Google Scholar]
- Gerlach E., Nees S., Becker B. F. The vascular endothelium: a survey of some newly evolving biochemical and physiological features. Basic Res Cardiol. 1985 Sep-Oct;80(5):459–474. doi: 10.1007/BF01907911. [DOI] [PubMed] [Google Scholar]
- HILTON S. M. A peripheral arterial conducting mechanism underlying dilatation of the femoral artery and concerned in functional vasodilatation in skeletal muscle. J Physiol. 1959 Dec;149:93–111. doi: 10.1113/jphysiol.1959.sp006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Kariniemi A. L., Hormia M., Linder E., Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982 Jul;47(1):60–66. [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Krogh A., Harrop G. A., Rehberg P. B. Studies on the physiology of capillaries: III. The innervation of the blood vessels in the hind legs of the frog. J Physiol. 1922 May 16;56(3-4):179–189. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. Studies on the capillariometer mechanism: I. The reaction to stimuli and the innervation of the blood vessels in the tongue of the frog. J Physiol. 1920 May 18;53(6):399–419. doi: 10.1113/jphysiol.1920.sp001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Nees S., Gerbes A. L., Gerlach E., Staubesand J. Isolation, identification, and continuous culture of coronary endothelial cells from guinea pig hearts. Eur J Cell Biol. 1981 Jun;24(2):287–297. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Duling B. R. Communication between feed arteries and microvessels in hamster striated muscle: segmental vascular responses are functionally coordinated. Circ Res. 1986 Sep;59(3):283–290. doi: 10.1161/01.res.59.3.283. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Duling B. R. Flow control among microvessels coordinated by intercellular conduction. Science. 1986 Nov 14;234(4778):868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Shiba H. Heaviside's "Bessel cable" as an electric model for flat simple epithelial cells with low resistive junctional membranes. J Theor Biol. 1971 Jan;30(1):59–68. doi: 10.1016/0022-5193(71)90036-1. [DOI] [PubMed] [Google Scholar]
- Spagnoli L. G., Villaschi S., Neri L., Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982 Jan 15;38(1):124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- Taugner R., Kirchheim H., Forssmann W. G. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res. 1984;235(2):319–325. doi: 10.1007/BF00217856. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]