Abstract
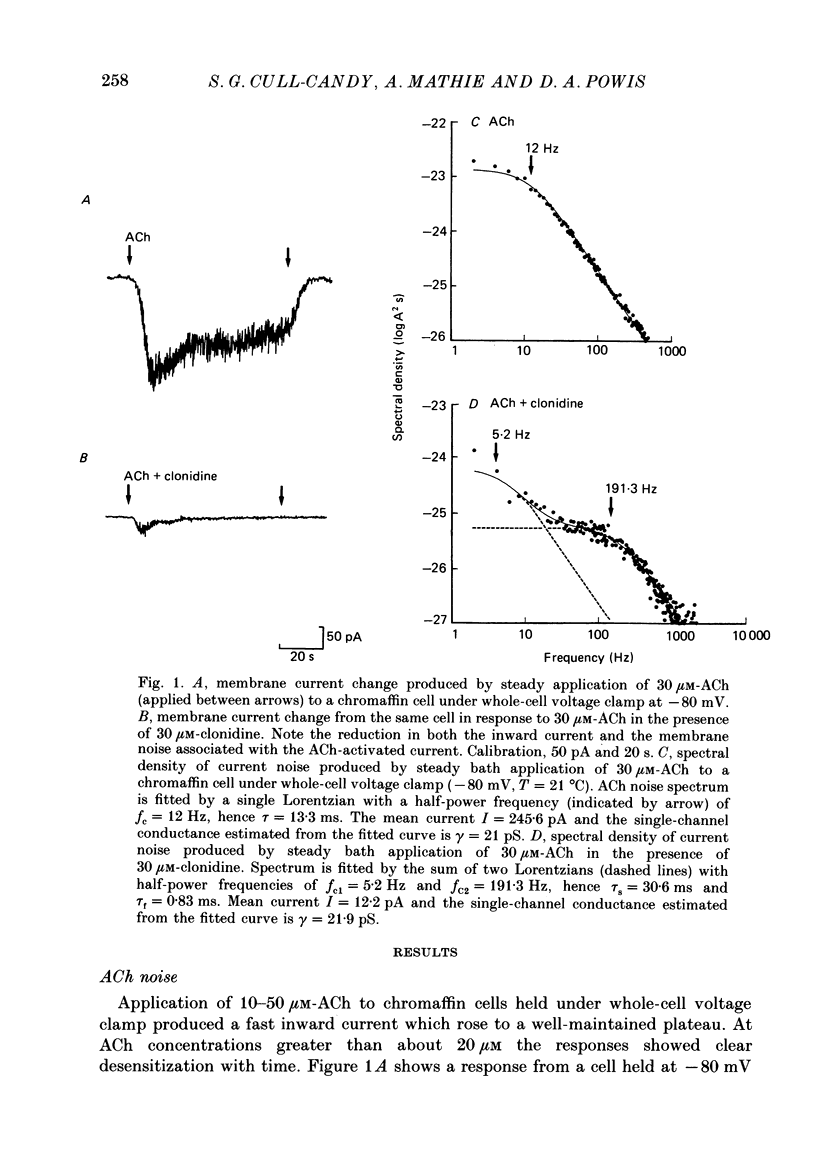
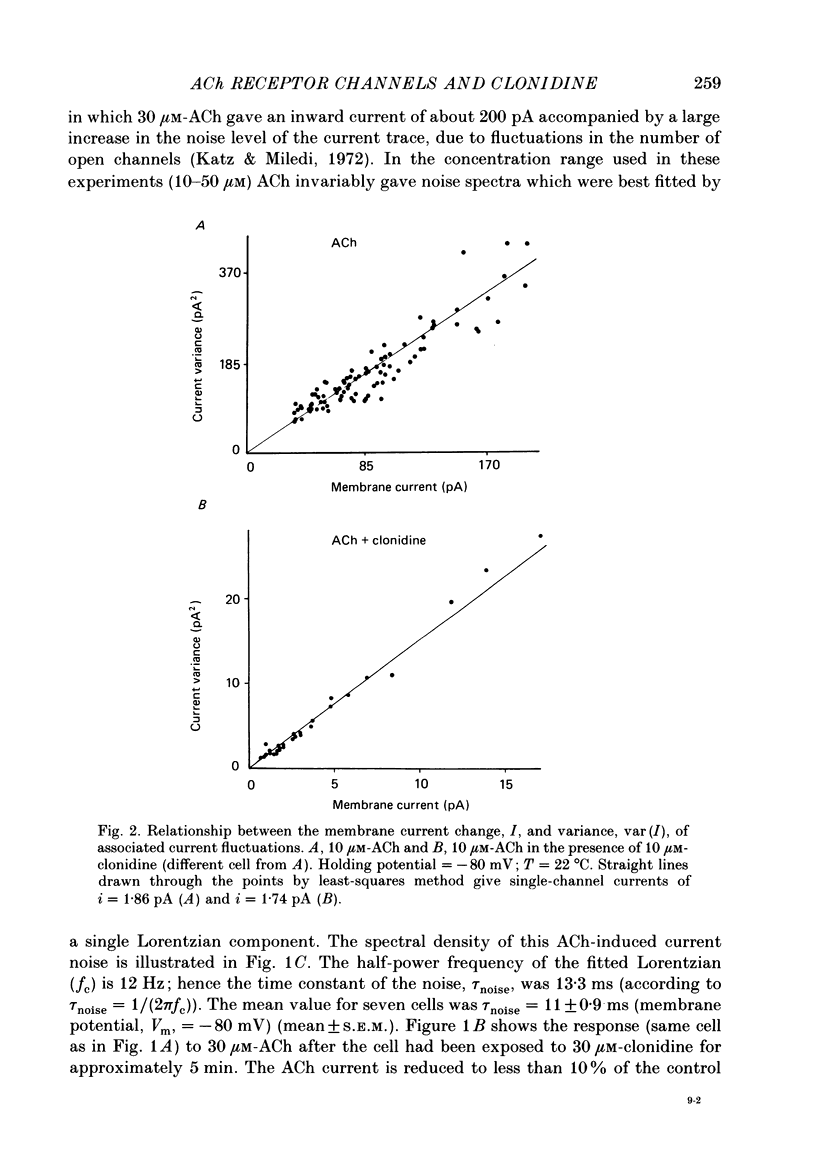
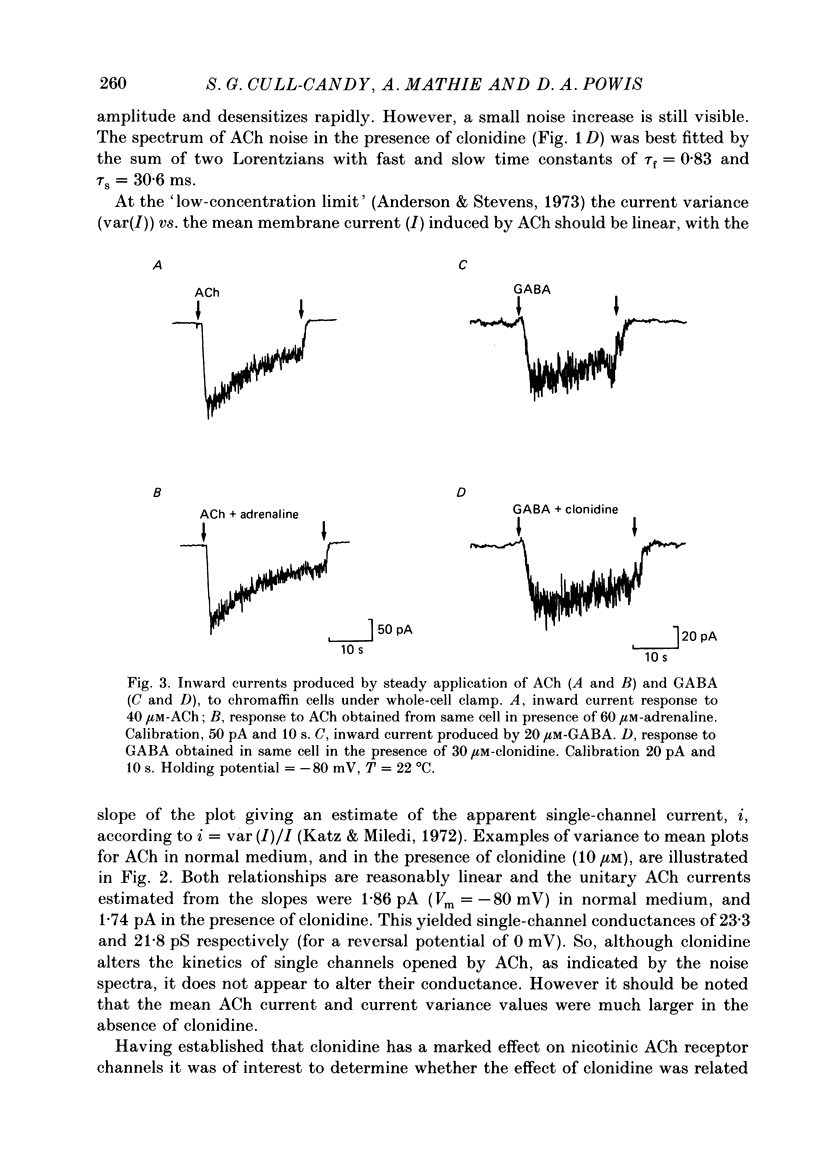
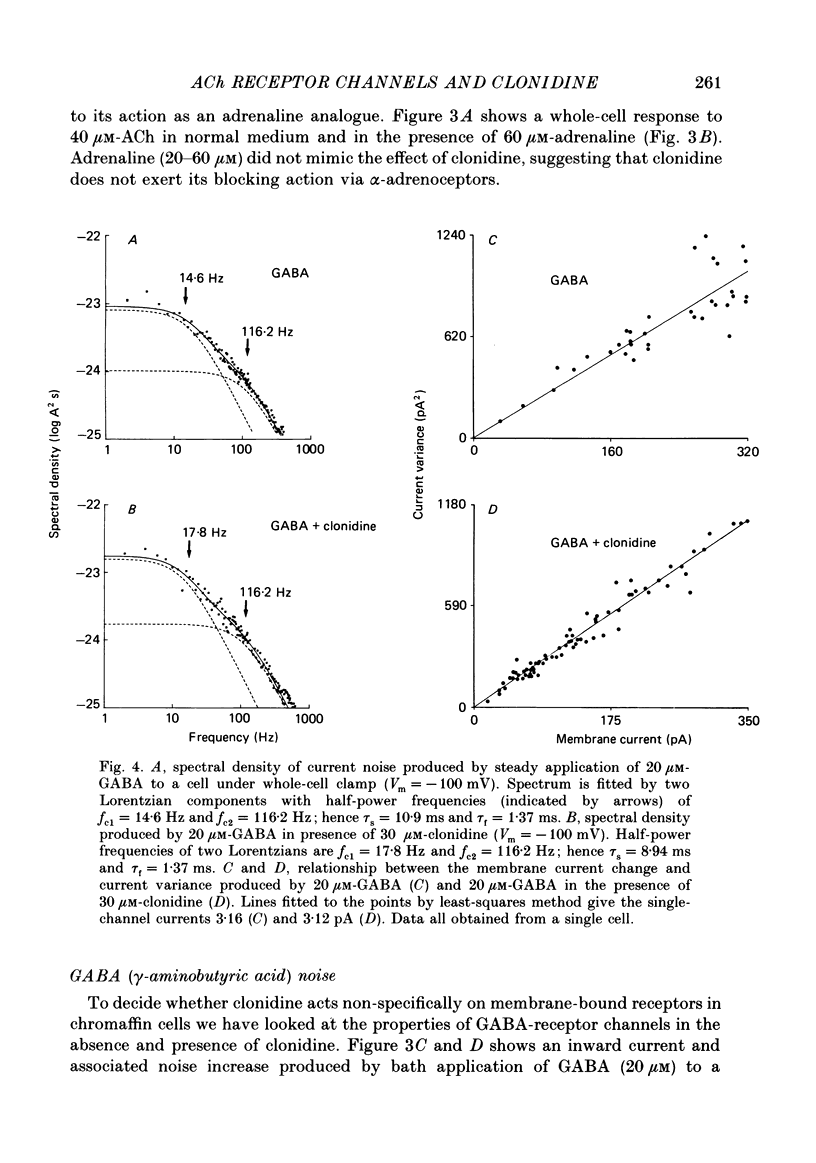
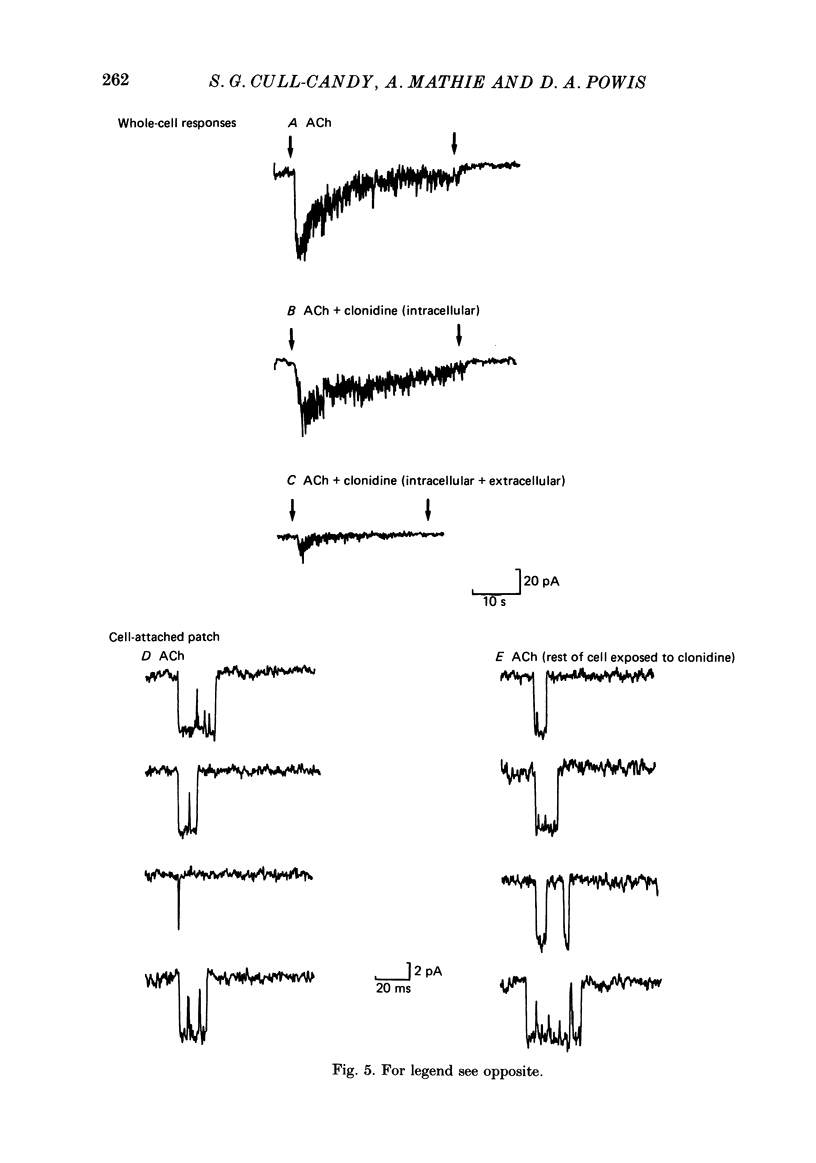
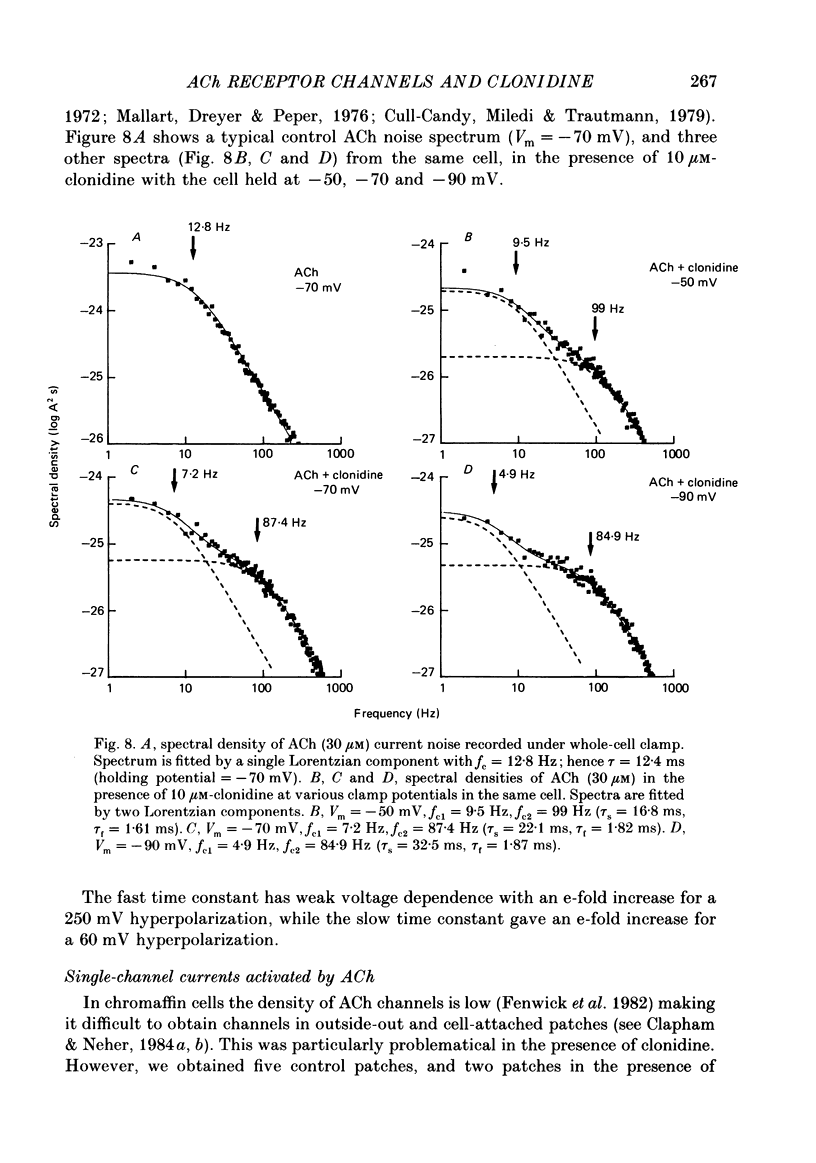
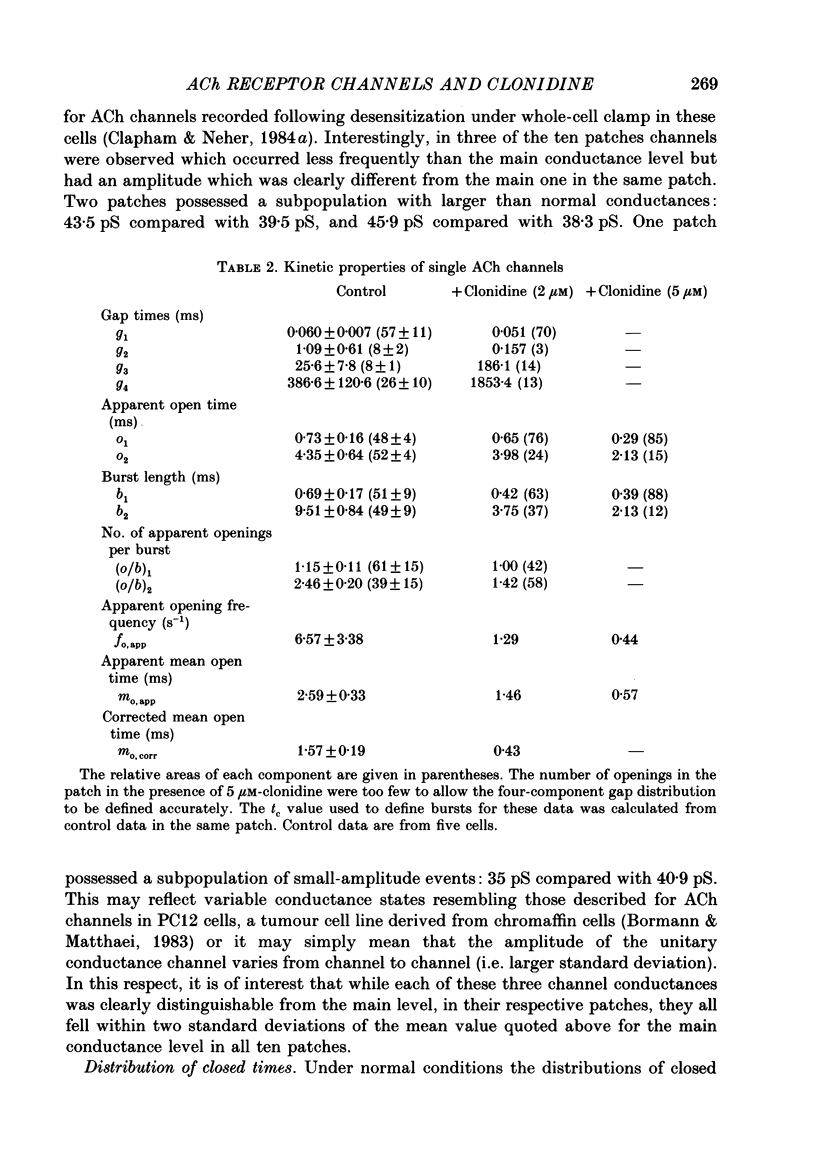
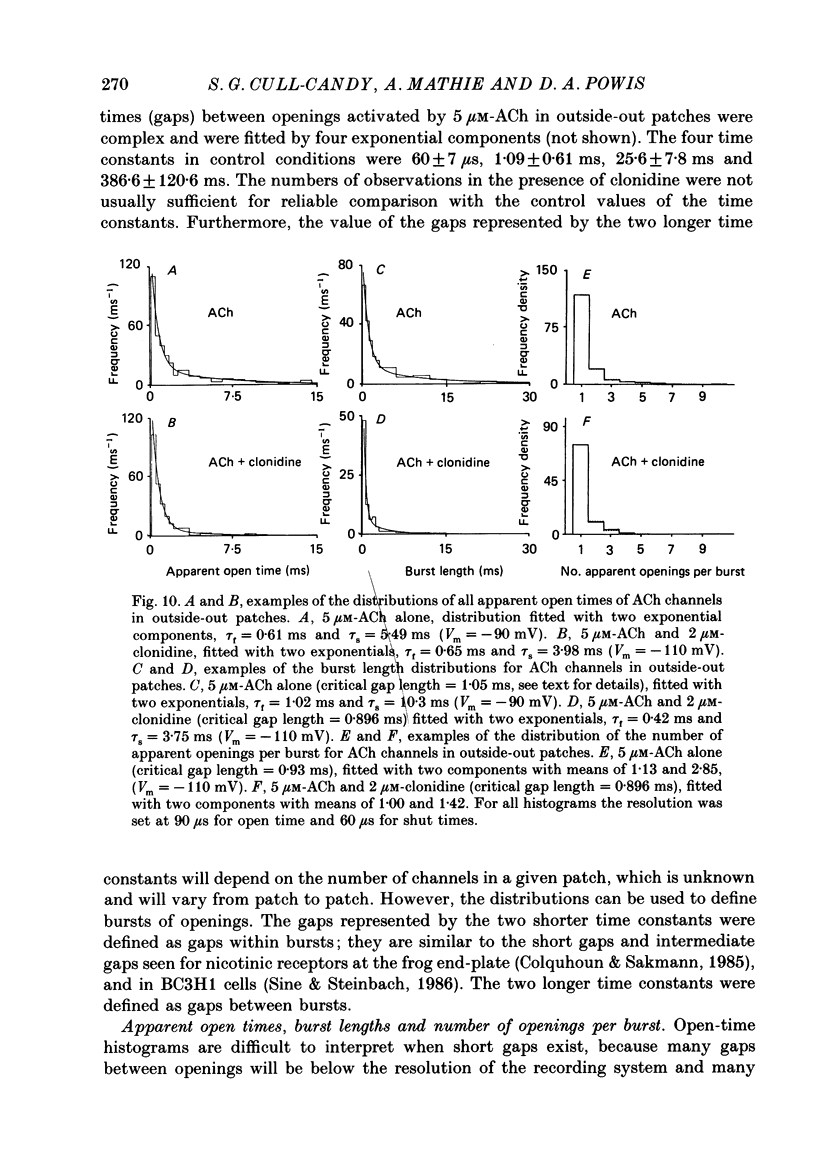
1. Acetylcholine (10-50 microM) applied to bovine chromaffin cells under whole-cell voltage clamp produced an inward current and an increase in the noise level of the current trace. At concentrations greater than or equal to 20 microM the ACh current desensitized with time. Spectral analysis of the ACh-induced increase in current noise showed that it could be fitted by a single Lorentzian component with a time constant, tau noise = 11 +/- 0.9 ms (Vm = -80 mV). 2. Clonidine (2-30 microM) markedly reduced the size of the ACh-induced whole-cell current, and altered the shape of the noise spectrum. In the presence of clonidine, ACh noise spectra were fitted by two Lorentzian components with time constants which varied with clonidine concentration. The single-channel conductance from noise (gamma = 24 pS) was unaltered by clonidine. 3. The reduction in the size of the whole-cell ACh current, produced by clonidine, was not mimicked by adrenaline (at concentrations up to 60 microM). GABA-induced whole-cell currents and spectra of GABA noise were also unchanged suggesting that the effect of clonidine was specific for the ACh receptor channel. 4. When applied intracellularly (from the patch pipette) clonidine had no apparent influence on the whole-cell ACh current. Furthermore, when clonidine was perfused over the cell surface at a concentration sufficient to block the whole-cell ACh response, single ACh channels could still be recorded under the patch-pipette tip (i.e. in cell-attached patches, not exposed to clonidine). 5. Clonidine block showed little voltage dependence; the peak ACh current remained linearly dependent on clamp potential. In addition, the fast and slow time constants derived from ACh noise spectra in the presence of clonidine did not show the sort of dependence on antagonist concentration expected for simple channel block; this suggests that clonidine has a complex blocking action. 6. Single ACh channels recorded in outside-out patches had a conductance of gamma = 39 +/- 0.7 pS, although a subpopulation of smaller and larger events also occurred in some patches. The mean single-channel conductance in outside-out patches was unaltered by clonidine. 7. Under normal conditions the kinetics of single ACh channels were more complex than suggested from noise analysis. Burst length distributions could be described by two exponential components with fast and slow time constants of tau f = 0.69 +/- 0.17 and tau s = 9.51 +/- 0.84 ms.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
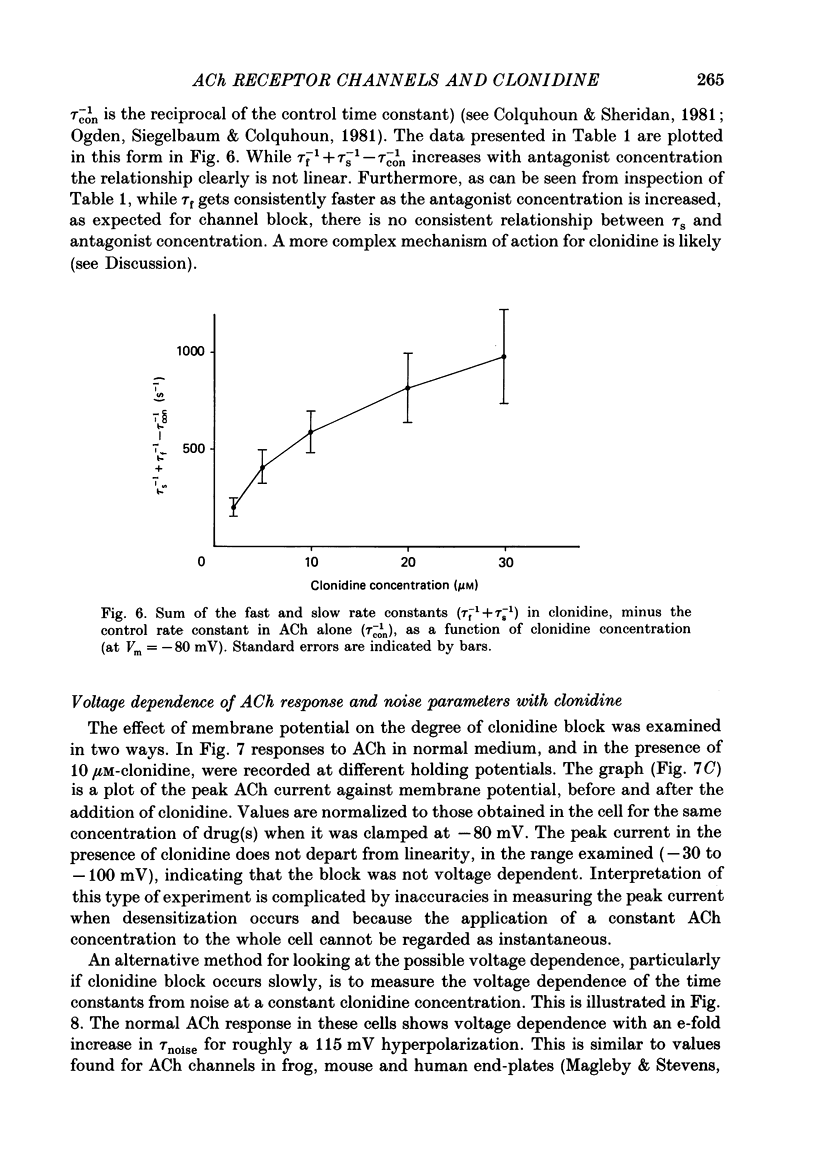
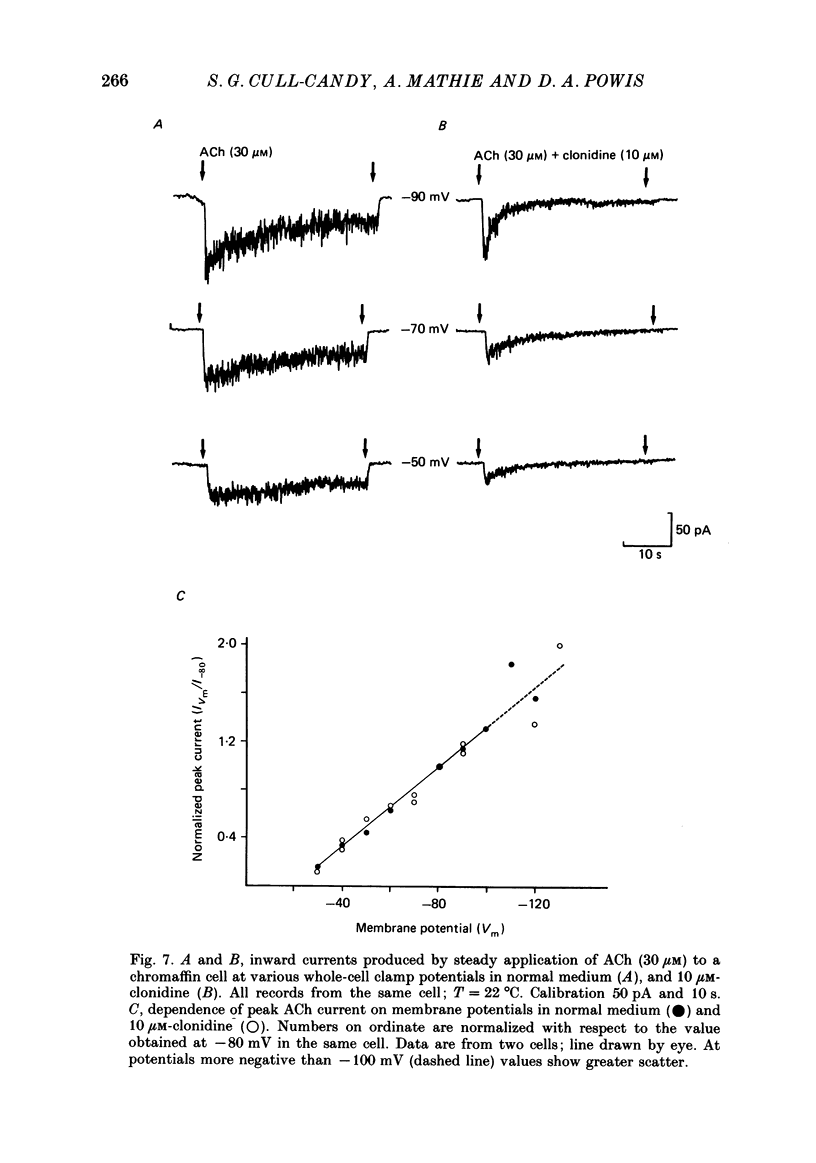
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Matthaei H. Three types of acetylcholine-induced single channel currents in clonal rat pheochromocytoma cells. Neurosci Lett. 1983 Sep 30;40(2):193–197. doi: 10.1016/0304-3940(83)90301-4. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A. K., Curry S. H., Jacobsen S. Localization of basic drugs in the submaxillary gland. Biochem Pharmacol. 1969 Oct;18(10):2323–2330. doi: 10.1016/0006-2952(69)90346-3. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett A. R., Story D. F. Release of 3H-adrenaline from an isolated intact preparation of the rabbit adrenal gland: no evidence for release modulatory alpha-adrenoreceptors. J Auton Pharmacol. 1982 Mar;2(1):25–34. doi: 10.1111/j.1474-8673.1982.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The modes of action of gallamine. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):181–203. doi: 10.1098/rspb.1981.0002. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Mathie A. Ion channels activated by acetylcholine and gamma-aminobutyric acid in freshly dissociated sympathetic neurones of the rat. Neurosci Lett. 1986 May 23;66(3):275–280. doi: 10.1016/0304-3940(86)90031-5. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. End-plate currents and acetylcholine noise at normal and myasthenic human end-plates. J Physiol. 1979 Feb;287:247–265. doi: 10.1113/jphysiol.1979.sp012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E. The kinetics of slow muscle acetylcholine-operated channels in the garter snake. J Physiol. 1981 Jan;310:159–190. doi: 10.1113/jphysiol.1981.sp013542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Walther C., Peper K. Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Noise analysis experiments with different agonists. Pflugers Arch. 1976 Oct 15;366(1):1–9. doi: 10.1007/BF02486555. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McKinnon D. Effects of pentobarbitone on acetylcholine-activated channels in mammalian muscle. Br J Pharmacol. 1985 May;85(1):229–235. doi: 10.1111/j.1476-5381.1985.tb08851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A., Zinder O. Alpha- and beta-receptor control of catecholamine secretion from isolated adrenal medulla cells. Cell Tissue Res. 1982;226(3):655–665. doi: 10.1007/BF00214792. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Rang H. P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br J Pharmacol. 1984 Jul;82(3):623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman Y., Boonyaviroj P. Suppression by noradrenaline of catecholamine secretion from adrenal medulla. Eur J Pharmacol. 1974 Oct;28(2):384–386. doi: 10.1016/0014-2999(74)90294-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of procaine on the action of acetylcholine at the neuromuscular junction. J Physiol. 1975 Jul;249(2):269–284. doi: 10.1113/jphysiol.1975.sp011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Ritchie A. K. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980 Oct;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Stimulus-secretion coupling in isolated bovine adrenal medullary cells. Q J Exp Physiol. 1983 Jan;68(1):123–143. doi: 10.1113/expphysiol.1983.sp002691. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Siegelbaum S. A., Colquhoun D. Block of acetylcholine-activated ion channels by an uncharged local anaesthetic. Nature. 1981 Feb 12;289(5798):596–598. doi: 10.1038/289596a0. [DOI] [PubMed] [Google Scholar]
- Powis D. A., Baker P. F. Alpha 2-adrenoceptors do not regulate catecholamine secretion by bovine adrenal medullary cells: a study with clonidine. Mol Pharmacol. 1986 Feb;29(2):134–141. [PubMed] [Google Scholar]
- Rang H. P., Colquhoun D., Rang H. P. The action of ganglionic blocking drugs on the synaptic responses of rat submandibular ganglion cells. Br J Pharmacol. 1982 Jan;75(1):151–168. doi: 10.1111/j.1476-5381.1982.tb08768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role L. W. Substance P modulation of acetylcholine-induced currents in embryonic chicken sympathetic and ciliary ganglion neurons. Proc Natl Acad Sci U S A. 1984 May;81(9):2924–2928. doi: 10.1073/pnas.81.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. The kinetics of local anesthetic blockade of end-plate channels. Biophys J. 1982 Mar;37(3):625–631. [PMC free article] [PubMed] [Google Scholar]
- Sakurai S., Wada A., Izumi F., Kobayashi H., Yanagihara N. Inhibition by alpha 2-adrenoceptor agonists of the secretion of catecholamines from isolated adrenal medullary cells. Naunyn Schmiedebergs Arch Pharmacol. 1983 Sep;324(1):15–19. doi: 10.1007/BF00647832. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Acetylcholine receptor activation by a site-selective ligand: nature of brief open and closed states in BC3H-1 cells. J Physiol. 1986 Jan;370:357–379. doi: 10.1113/jphysiol.1986.sp015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A., Sakurai S., Kobayashi H., Yanagihara N., Izumi F. alpha 2-Adrenergic receptors inhibit catecholamine secretion from bovine adrenal medulla. Brain Res. 1982 Dec 2;252(1):189–191. doi: 10.1016/0006-8993(82)90997-0. [DOI] [PubMed] [Google Scholar]


