Abstract
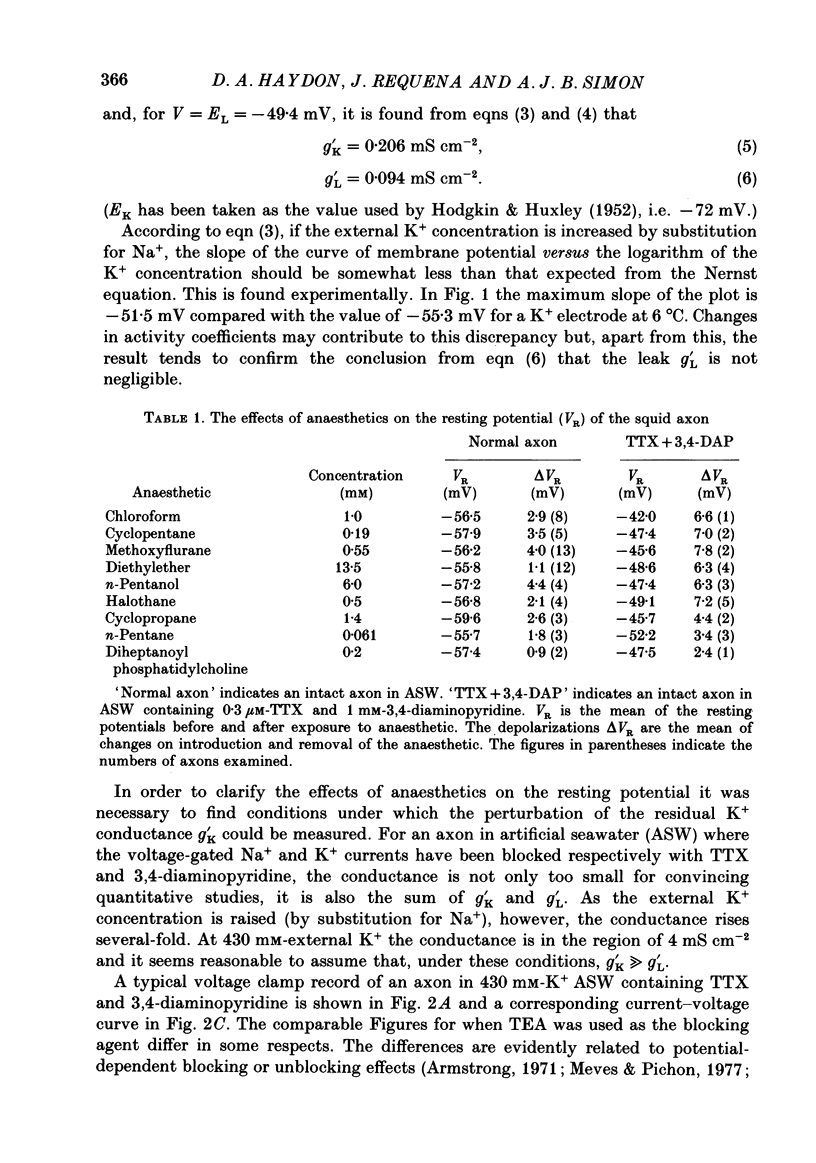
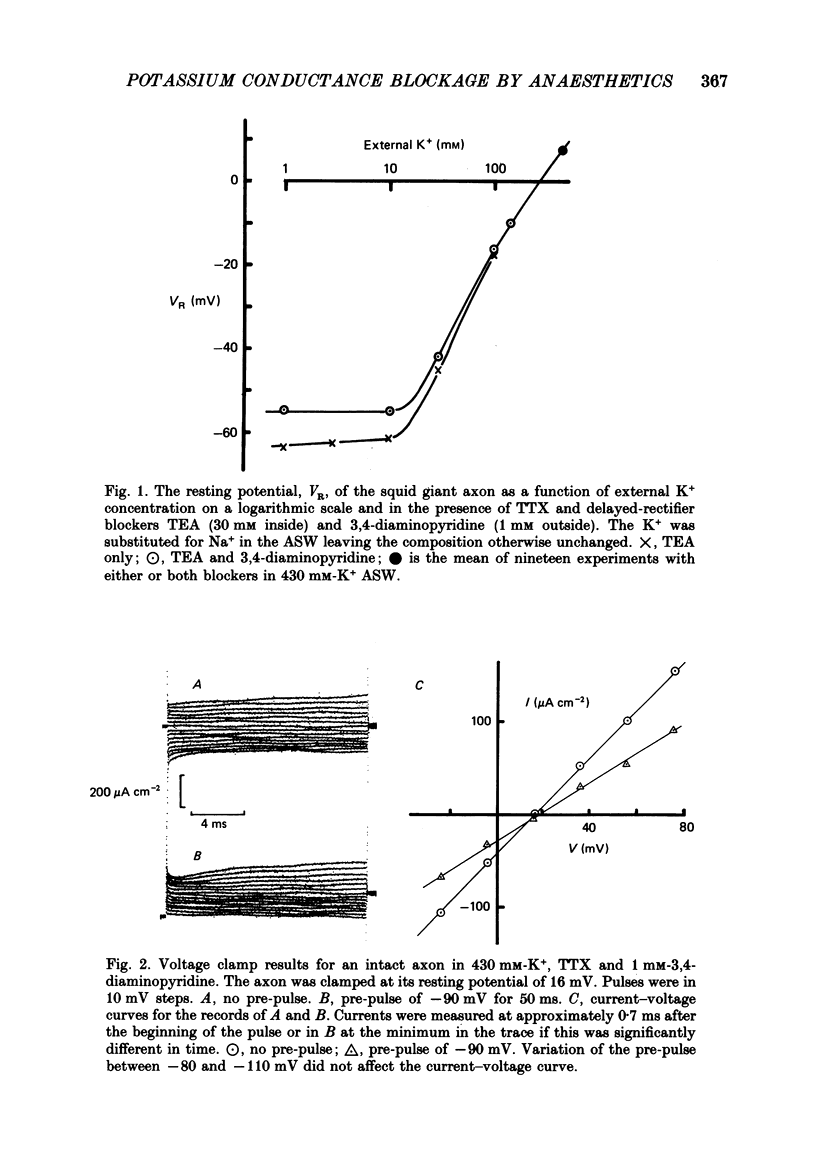
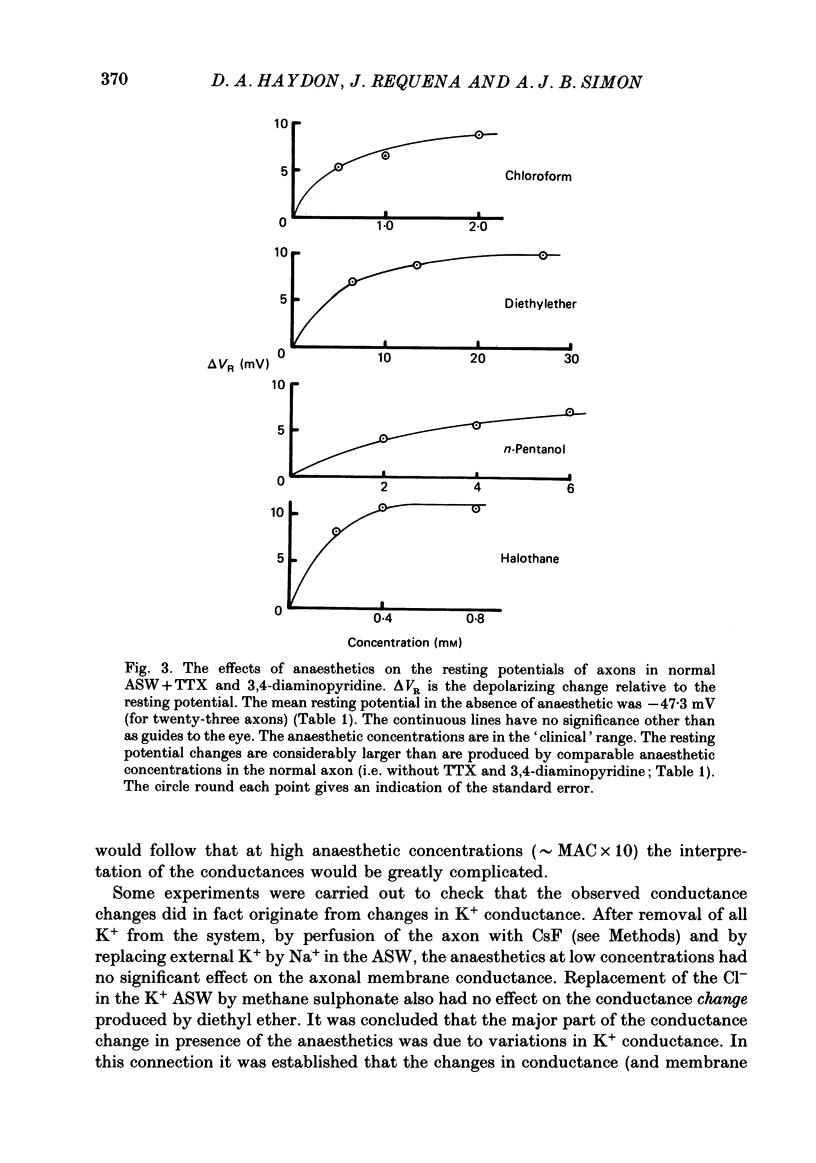
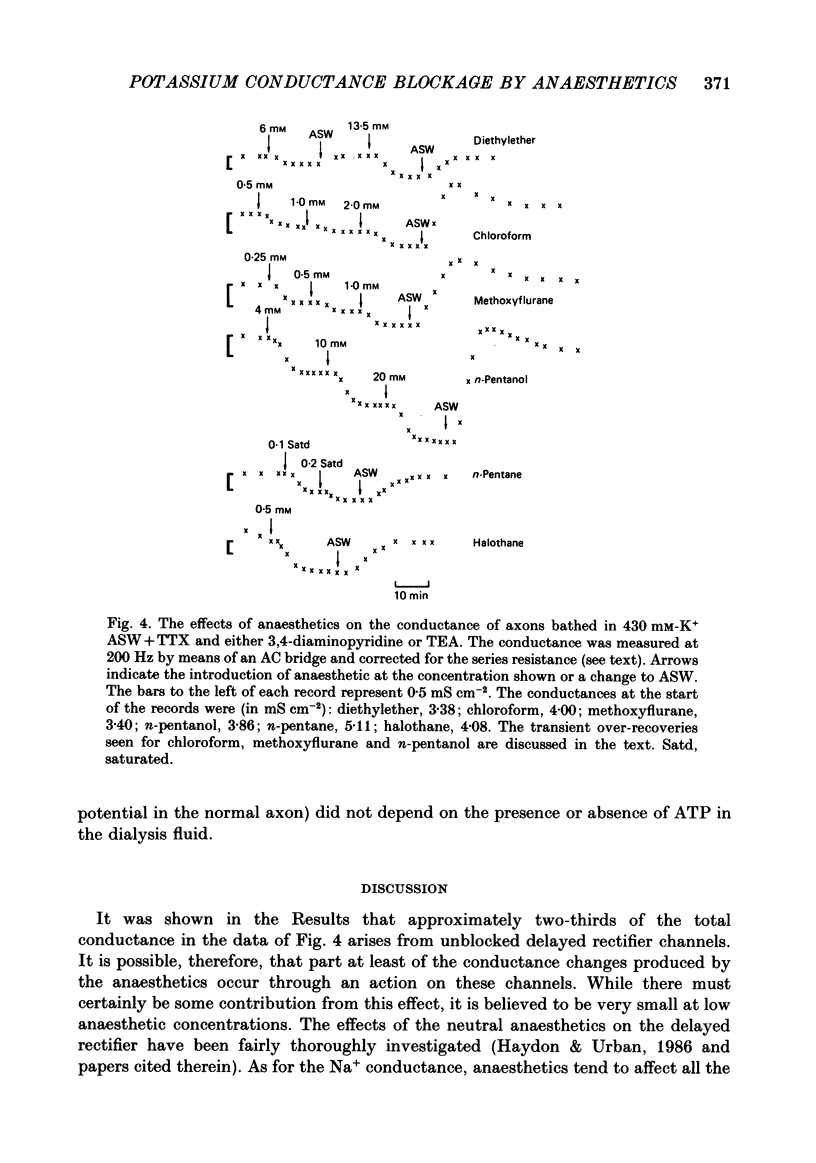
1. The effects of some neutral clinical and experimental general anaesthetics on the resting potential of normal squid axons and squid axons exposed to tetrodotoxin and 3,4-diaminopyridine have been studied. 2. Depolarizations of 1-4 mV were produced by all the anaesthetics at 'clinical' concentrations in the normal axon. Larger depolarizations (5-11 mV) were produced by the same anaesthetic concentrations in axons exposed to tetrodotoxin and 3,4-diaminopyridine. 3. The conductance of axons exposed to tetrodotoxin and either tetraethyl-ammonium or 3,4-diaminopyridine in zero Na+, 430 mM-K+ artificial sea water was examined by voltage clamp and AC bridge techniques. 4. The evidence that this conductance is due predominantly to K+ is discussed. 5. Pre-pulse protocols under voltage clamp have been used to show that part of this conductance arises from the incompletely blocked delayed rectifier. 6. Substantial reductions in this conductance are produced by anaesthetics at 'clinical' concentrations. 7. It is concluded that there is a component of the K+ conductance of the resting squid axon other than the Hodgkin-Huxley delayed rectifier which is extremely sensitive to anaesthetics and which to an appreciable extent determines the resting potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983 Mar;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. C. Is the K permeability of the resting membrane controlled by the excitable K channel? Biophys J. 1986 Dec;50(6):1095–1100. doi: 10.1016/S0006-3495(86)83553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M. The asymmetrical effects of some ionized n-octyl derivatives on the sodium current of the giant axon of Loligo forbesi. J Physiol. 1984 May;350:429–445. doi: 10.1113/jphysiol.1984.sp015210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Urban B. W. Some effects of aliphatic hydrocarbons on the electrical capacity and ionic currents of the squid giant axon membrane. J Physiol. 1980 Dec;309:229–245. doi: 10.1113/jphysiol.1980.sp013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. J Physiol. 1983 May;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The actions of some general anaesthetics on the potassium current of the squid giant axon. J Physiol. 1986 Apr;373:311–327. doi: 10.1113/jphysiol.1986.sp016049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978 Jun;22(3):507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevcik C. Differences between the actions of thiopental and pentobarbital in squid giant axons. J Pharmacol Exp Ther. 1980 Sep;214(3):657–663. [PubMed] [Google Scholar]
- Urban B. W., Haydon D. A. The actions of halogenated ethers on the ionic currents of the squid giant axon. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):13–26. doi: 10.1098/rspb.1987.0032. [DOI] [PubMed] [Google Scholar]


