Abstract
1. The preceding paper (Van der Kloot, 1988) described a method for estimating the timing of quantal releases during an end-plate current. This period of elevated quantal release is called the early release period or ERP (Barrett & Stevens, 1972b). In the present paper, this deconvolution method is used to study the effects of varying quantal output by extracellular ions, stimulus patterns and drugs. 2. The data were obtained by voltage clamping end-plates in low-Ca2+ high-Mg2+ solutions, or in solutions containing tubocurarine (measuring the decay of the miniature end-plate currents (MEPCs) before curarization and assuming a value for MEPC amplitude after curarization). Data were also obtained by extracellular recording in Ca2+-free solution, using a recording pipette filled with CaCl2 and regulating Ca2+ release with a bias current. The three approaches led to similar conclusions. 3. Quantal release rose during the ERP along a sigmoid curve and reached a maximum after about 1.4 ms at 10 degrees C. This is called the time to peak. Quantal release then fell, following an exponential time course with a time constant of about 1.2 ms (10 degrees C). This is called the time constant for decline. 4. The ERP was followed by further, elevated quantal release, at a much lower rate, which declined over a longer time course. This is called late release. The magnitude of late release appears to be almost independent of the magnitude of release during the ERP, although the deconvolution method is a poor one for determining late release. The remainder of the results therefore focus on the ERP. 5. Increasing [Ca2+]o increased quantal output, and the rate of quantal output. It did not change the time to peak or the time constant of decline. Similarly, replacing Ca2+ with Sr2+ did not alter the time course of the ERP. 6. Two-pulse facilitation increased quantal output without changing the time to peak or the time constant of decline. 7. Quantal output was enhanced still more following a brief series of repetitive nerve stimulations. There was a lengthening of the time to peak; there was no change in the decline. The depression produced by longer series of repetitive stimulations did not change the time course of the ERP. 8. 4-Aminopyridine (4-AP) and dimethylsulphoxide (DMSO) increased quantal output and lengthened the time to peak, without altering the time constant for decline. 9. Adenosine decreased quantal output without altering the time course of the ERP.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



















Selected References
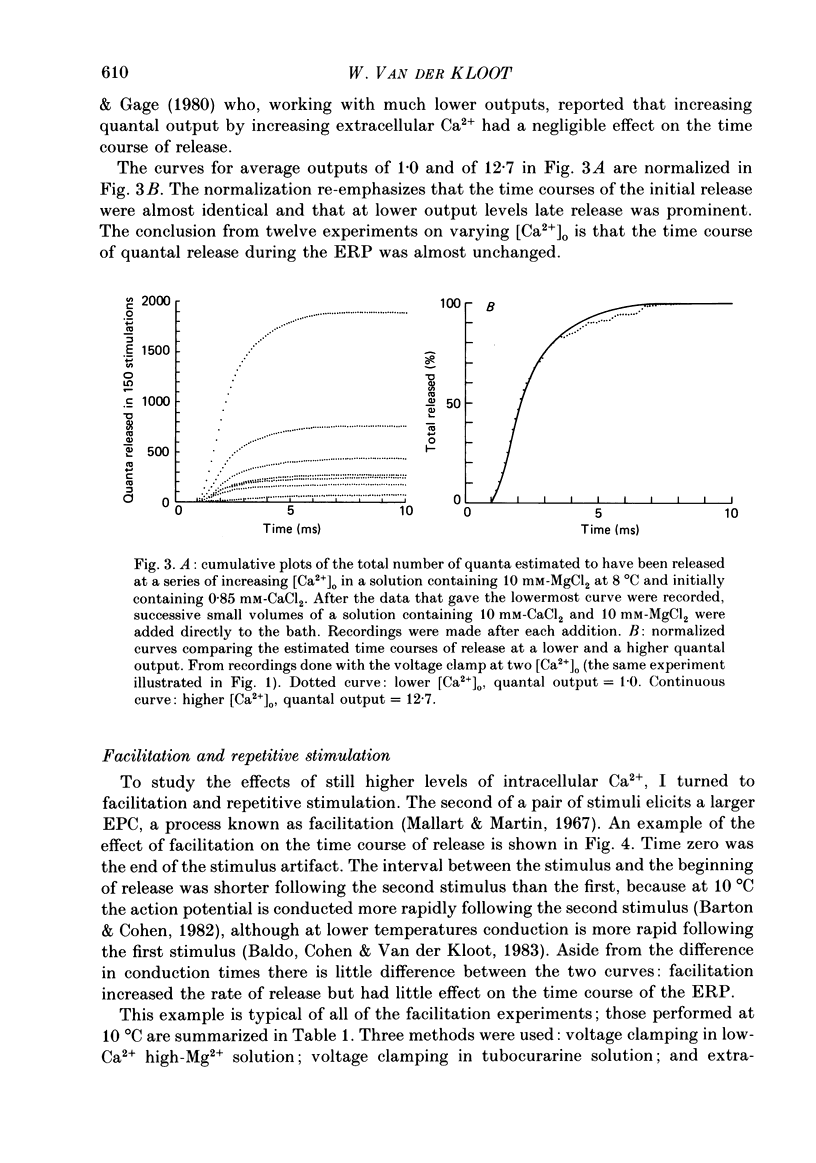
These references are in PubMed. This may not be the complete list of references from this article.
- Baldo G. J., Cohen I. S., Van der Kloot W. Estimating the time course of evoked quantal release at the frog neuromuscular junction using end-plate current latencies. J Physiol. 1986 May;374:503–513. doi: 10.1113/jphysiol.1986.sp016094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo G. J., Cohen I. S., Van der Kloot W. Facilitation and the conduction of the nerve action potential at the frog neuromuscular junction. Pflugers Arch. 1983 Nov;399(3):161–165. doi: 10.1007/BF00656709. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton S. B., Cohen I. S. Facilitation and impulse propagation failure at the frog neuromuscular junction. Pflugers Arch. 1982 Feb;392(4):327–334. doi: 10.1007/BF00581627. [DOI] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., van der Kloot W., Attwell D. The timing of channel opening during miniature end-plate currents. Brain Res. 1981 Oct 26;223(1):185–189. doi: 10.1016/0006-8993(81)90821-0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant N. N., Marshall I. G. The effects of 3,4-diaminopyridine on spontaneous and evoked transmitter release at the frog neuromuscular juction [proceedings]. J Physiol. 1978 Jul;280:21P–21P. [PubMed] [Google Scholar]
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Armstrong C. M. Miniature end-plate currents in voltage-clamped muscle fibre. Nature. 1968 Apr 27;218(5139):363–365. doi: 10.1038/218363b0. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefevre J., Kretsinger R. H. Formation of calcium-parvalbumin complex during contraction. A source of "unexplained heat"? Adv Exp Med Biol. 1984;170:573–579. doi: 10.1007/978-1-4684-4703-3_52. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Hirst G. D. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol. 1972 Aug;224(3):629–645. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner M. M., Narahashi T. Effects of edrophonium on end-plate currents in frog skeletal muscle. Eur J Pharmacol. 1974 Mar;25(3):362–371. doi: 10.1016/0014-2999(74)90266-0. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KUNO M. QUANTAL COMPONENTS OF EXCITATORY SYNAPTIC POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:81–99. doi: 10.1113/jphysiol.1964.sp007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H., Thesleff S. The mode of action of 4-aminopyridine and guanidine on transmitter release from motor nerve terminals. Eur J Pharmacol. 1977 Apr 21;42(4):411–412. doi: 10.1016/0014-2999(77)90176-5. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon J. G., Saint D. A., Quastel D. M. The actions of dimethyl sulfoxide on neuromuscular transmission. Mol Pharmacol. 1986 Dec;30(6):631–638. [PubMed] [Google Scholar]
- Molgo J., Lemeignan M., Lechat P. Effects of 4-aminopyridine at the frog neuromuscular junction. J Pharmacol Exp Ther. 1977 Dec;203(3):653–663. [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol. 1987 Mar;384:571–585. doi: 10.1113/jphysiol.1987.sp016470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockbridge N., Moore J. W. Dynamics of intracellular calcium and its possible relationship to phasic transmitter release and facilitation at the frog neuromuscular junction. J Neurosci. 1984 Mar;4(3):803–811. doi: 10.1523/JNEUROSCI.04-03-00803.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. Estimating the timing of quantal releases during end-plate currents at the frog neuromuscular junction. J Physiol. 1988 Aug;402:595–603. doi: 10.1113/jphysiol.1988.sp017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Motta G. E., Córdoba F., de León M., del Castillo J. Inhibitory action of high formamide concentrations on excitation-contraction coupling in skeletal muscle. J Neurosci Res. 1982;7(2):163–178. doi: 10.1002/jnr.490070208. [DOI] [PubMed] [Google Scholar]


