Abstract
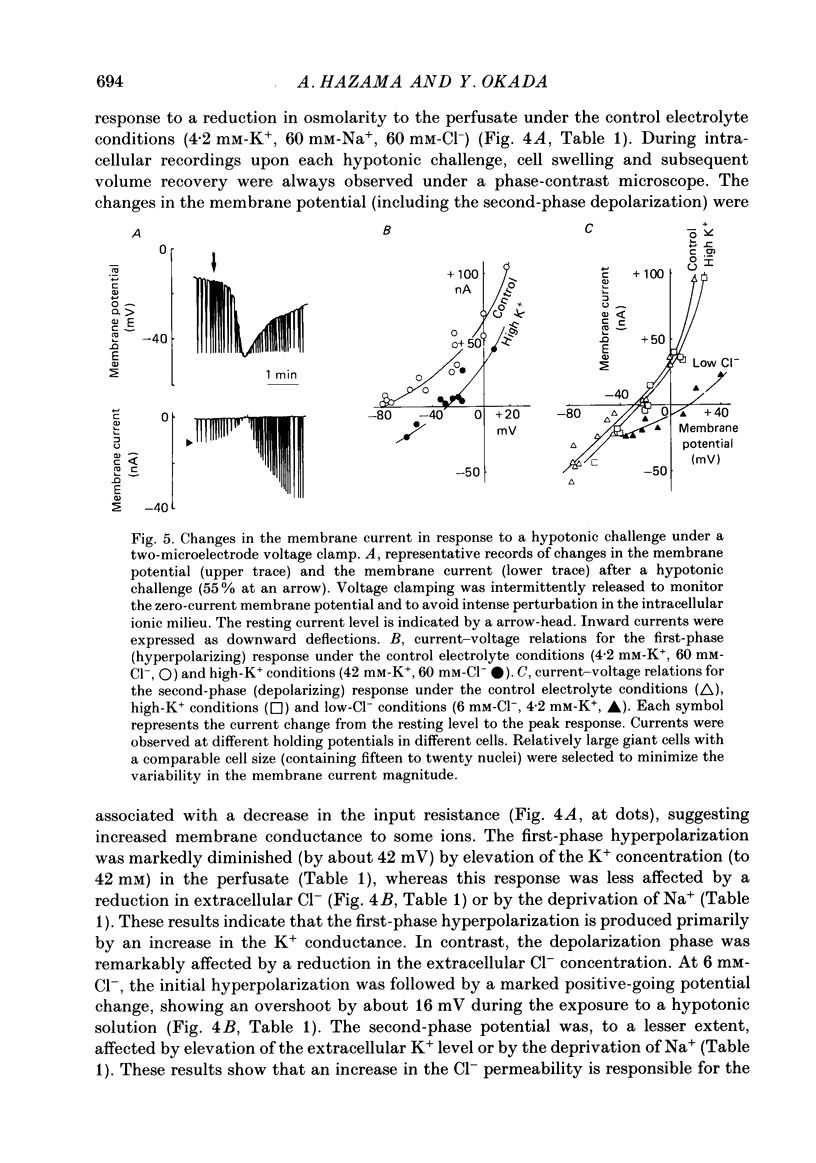
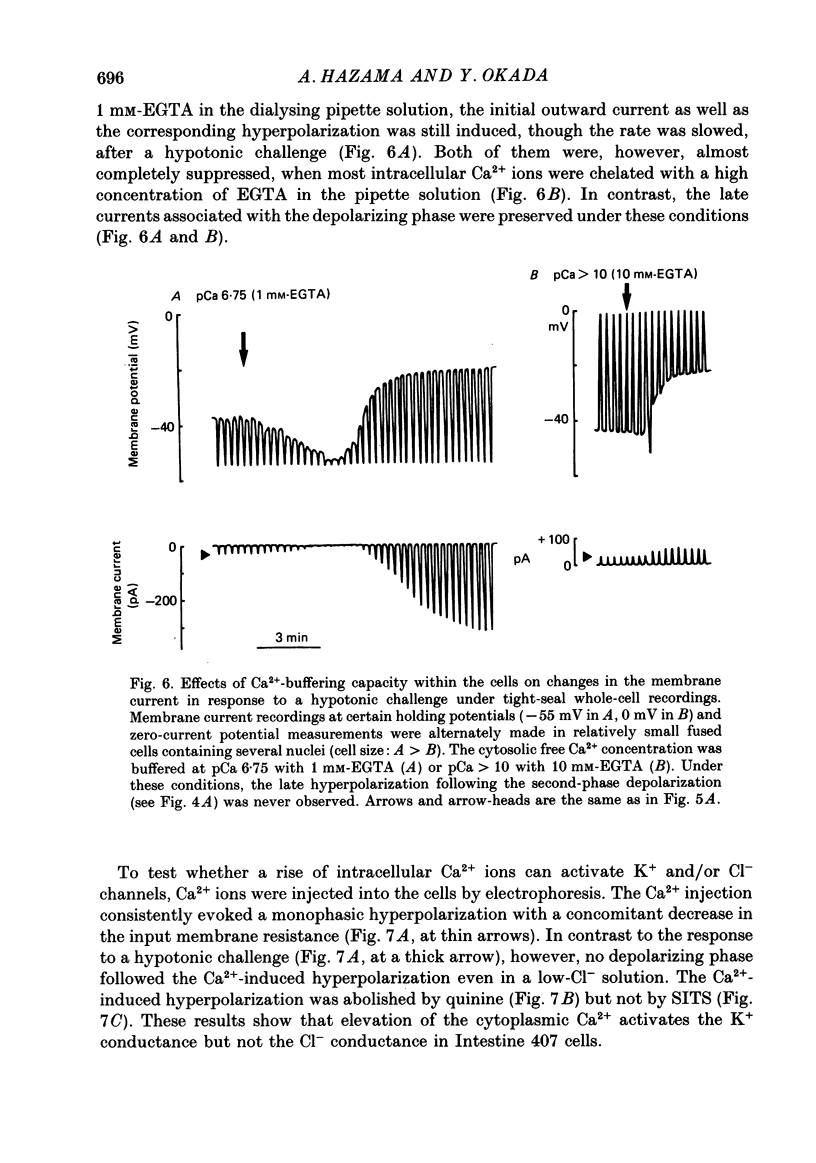
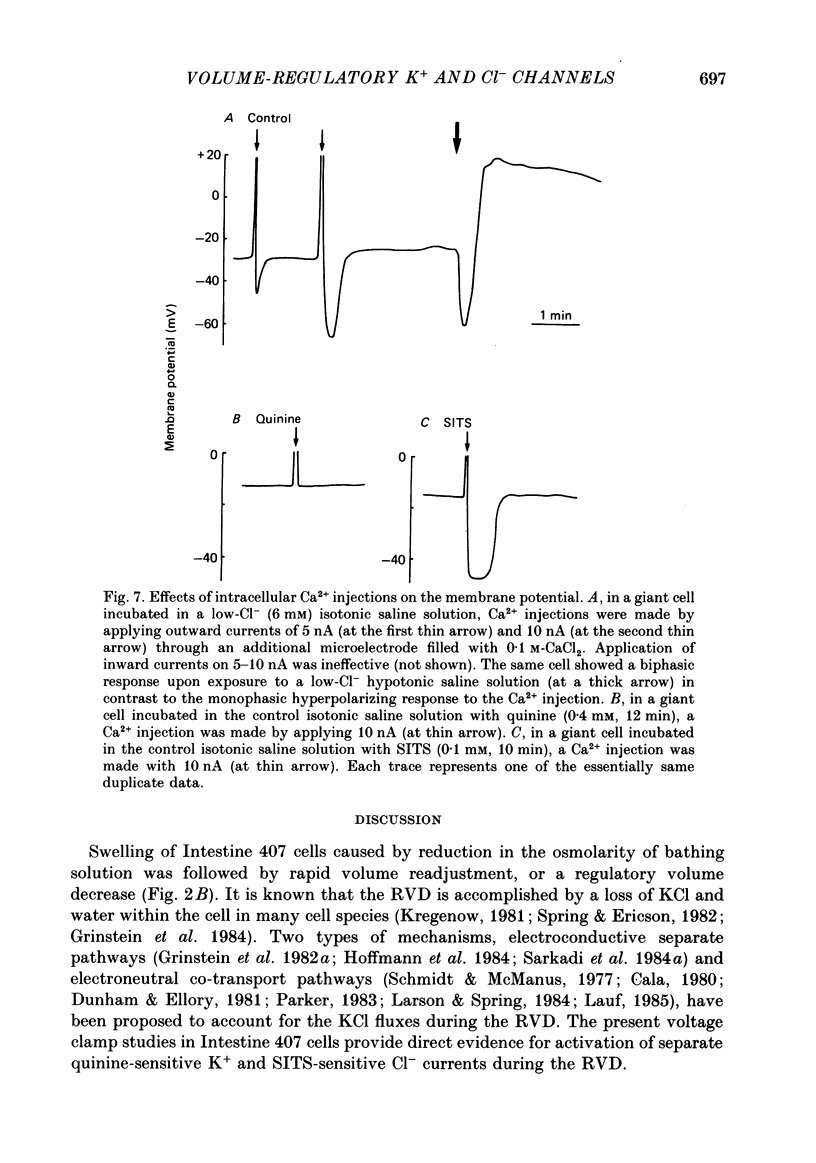
1. During exposure to a hypotonic solution, cultured human epithelial cells (Intestine 407) exhibited a regulatory volume decrease (RVD) after initial osmotic swelling. 2. The volume readjustment was slowed by elevating the extracellular K+ concentration and facilitated by reducing the extracellular Cl- concentration. Not only putative K+ channel blockers, quinine and Ba2+, but also a stilbene derivative Cl- channel blocker (SITS) inhibited the RVD. 3. The volume recovery of hypoosmotically swollen cells was very much suppressed by the deprivation of extracellular Ca2+ ions or by chelation of cytosolic Ca2+ ions with Quin-2 loaded within the cells. 4. Biphasic membrane potential changes were associated with the RVD process at low extracellular K+ and Cl- concentrations. The initial hyperpolarizing response was inhibited by quinine and Ba2+, whereas the late depolarizing response was inhibited by SITS. The deprivation of extracellular Ca2+ inhibited the initial hyperpolarizing phase but not the late depolarizing phase. 5. Two-microelectrode voltage clamp studies showed that the initial hyperpolarization and late depolarization were associated with quinine-sensitive outward currents and SITS-sensitive inward currents, respectively. The reversal potentials estimated from the current-voltage curves were about -80 mV for the initial response and -27 mV for the late response. Tenfold changes in the K+ and Cl- concentrations shifted these reversal potentials by 50 mV for the initial response and by 42 mV for the late response. 6. Under whole-cell recordings, similar current changes were observed in the cells exposed to a hypotonic solution, when the intracellular Ca2+ ions were moderately buffered with 1 mM-EGTA in the dialysing solution filled in a patch pipette. When most Ca2+ ions were chelated with 10 mM-EGTA in the pipette solution, the initial outward current as well as the corresponding hyperpolarization was suppressed, but the late current associated with the depolarizing phase was preserved. 7. Intracellular Ca2+ injections induced an increase in the quinine-sensitive K+ conductance but failed to activate the Cl- conductance. 8. It is concluded that both K+ and Cl- channels are involved in the regulatory volume decrease, and that the former channel is exclusively activated by elevation of the cytosolic Ca2+ concentration in the epithelial cells.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong W. M., Byrd B. J., Cohen E. S., Cohen S. J., Hamang P. H., Myers C. J. Osmotically induced electrical changes in isolated bullfrog small intestine. Biochim Biophys Acta. 1975 Aug 5;401(1):137–151. doi: 10.1016/0005-2736(75)90348-x. [DOI] [PubMed] [Google Scholar]
- Armstrong W. M., Musselman D. L., Reitzug H. C. Sodium, potassium, and water content of isolated bullfrog small intestinal epithelia. Am J Physiol. 1970 Oct;219(4):1023–1026. doi: 10.1152/ajplegacy.1970.219.4.1023. [DOI] [PubMed] [Google Scholar]
- Cala P. M. Cell volume regulation by Amphiuma red blood cells. The role of Ca+2 as a modulator of alkali metal/H+ exchange. J Gen Physiol. 1983 Dec;82(6):761–784. doi: 10.1085/jgp.82.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala P. M., Mandel L. J., Murphy E. Volume regulation by Amphiuma red blood cells: cytosolic free Ca and alkali metal-H exchange. Am J Physiol. 1986 Mar;250(3 Pt 1):C423–C429. doi: 10.1152/ajpcell.1986.250.3.C423. [DOI] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by Amphiuma red blood cells. The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol. 1980 Dec;76(6):683–708. doi: 10.1085/jgp.76.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Costa P. M., Fernandes P. L., Ferreira H. G., Ferreira K. T., Giraldez F. Effects of cell volume changes on membrane ionic permeabilities and sodium transport in frog skin (Rana ridibunda). J Physiol. 1987 Dec;393:1–17. doi: 10.1113/jphysiol.1987.sp016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csáky T. Z., Esposito G. Osmotic swelling of intestinal epithelial cells during active sugar transport. Am J Physiol. 1969 Sep;217(3):753–755. doi: 10.1152/ajplegacy.1969.217.3.753. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Interactions of sodium transport, cell volume, and calcium in frog urinary bladder. J Gen Physiol. 1987 May;89(5):687–702. doi: 10.1085/jgp.89.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B., Ellory J. C. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981 Sep;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink J. G., Deierkauf M. The effect of quin2 on chemotaxis by polymorphonuclear leukocytes. Biochim Biophys Acta. 1985 Sep 30;846(3):364–369. doi: 10.1016/0167-4889(85)90007-2. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A., Tan Y. P., Trautmann A. Blockage of Ca-activated Cl conductance by furosemide in rat lacrimal glands. Pflugers Arch. 1986 Jan;406(1):65–68. doi: 10.1007/BF00582955. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Ullrich S., Wollheim C. B., Petersen O. H. Quinine inhibits Ca2+-independent K+ channels whereas tetraethylammonium inhibits Ca2+-activated K+ channels in insulin-secreting cells. FEBS Lett. 1985 Jun 3;185(1):4–8. doi: 10.1016/0014-5793(85)80729-8. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Spring K. R. Involvement of calcium and cytoskeleton in gallbladder epithelial cell volume regulation. Am J Physiol. 1985 Jan;248(1 Pt 1):C27–C36. doi: 10.1152/ajpcell.1985.248.1.C27. [DOI] [PubMed] [Google Scholar]
- Geck P., Heinz E. The Na-K-2Cl cotransport system. J Membr Biol. 1986;91(2):97–105. doi: 10.1007/BF01925787. [DOI] [PubMed] [Google Scholar]
- Gitter A. H., Beyenbach K. W., Christine C. W., Gross P., Minuth W. W., Frömter E. High-conductance K+ channel in apical membranes of principal cells cultured from rabbit renal cortical collecting duct anlagen. Pflugers Arch. 1987 Mar;408(3):282–290. doi: 10.1007/BF02181471. [DOI] [PubMed] [Google Scholar]
- Gregg E. C., Steidley K. D. Electrical counting and sizing of mammalian cells in suspension. Biophys J. 1965 Jul;5(4):393–405. doi: 10.1016/S0006-3495(65)86724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Dupre A., Rothstein A. Volume-induced increase of anion permeability in human lymphocytes. J Gen Physiol. 1982 Dec;80(6):801–823. doi: 10.1085/jgp.80.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Rothstein A. Activation of Na+/H+ exchange in lymphocytes by osmotically induced volume changes and by cytoplasmic acidification. J Gen Physiol. 1983 Nov;82(5):619–638. doi: 10.1085/jgp.82.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Dupre A., Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982 May;79(5):849–868. doi: 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hazama A., Yada T., Okada Y. HeLa cells have histamine H1-receptors which mediate activation of the K+ conductance. Biochim Biophys Acta. 1985 May 30;845(2):249–253. doi: 10.1016/0167-4889(85)90183-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Lambert I. H., Simonsen L. O. Separate, Ca2+-activated K+ and Cl- transport pathways in Ehrlich ascites tumor cells. J Membr Biol. 1986;91(3):227–244. doi: 10.1007/BF01868816. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O., Lambert I. H. Volume-induced increase of K+ and Cl- permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+. J Membr Biol. 1984;78(3):211–222. doi: 10.1007/BF01925969. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Sjøholm C., Simonsen L. O. Na+,Cl- cotransport in Ehrlich ascites tumor cells activated during volume regulation (regulatory volume increase). J Membr Biol. 1983;76(3):269–280. doi: 10.1007/BF01870369. [DOI] [PubMed] [Google Scholar]
- Humphreys M. H. Inhibition of NaCl absorption from perfused rat ileum by furosemide. Am J Physiol. 1976 Jun;230(6):1517–1523. doi: 10.1152/ajplegacy.1976.230.6.1517. [DOI] [PubMed] [Google Scholar]
- Hunter M., Lopes A. G., Boulpaep E. L., Giebisch G. H. Single channel recordings of calcium-activated potassium channels in the apical membrane of rabbit cortical collecting tubules. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4237–4239. doi: 10.1073/pnas.81.13.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. L., Halm D. R., Dawson D. C. Active sodium transport by turtle colon via an electrogenic Na-K exchange pump. Nature. 1980 Sep 18;287(5779):237–239. doi: 10.1038/287237a0. [DOI] [PubMed] [Google Scholar]
- Kleinzeller A., Nedvídková J., Knotková A. Effect of saline osmolarity on the steady-state level of water and electrolytes in kidney cortex cells. Biochim Biophys Acta. 1967 May 2;135(2):286–299. doi: 10.1016/0005-2736(67)90122-8. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Kesteven N. T. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. Osmoregulatory salt transporting mechanisms: control of cell volume in anisotonic media. Annu Rev Physiol. 1981;43:493–505. doi: 10.1146/annurev.ph.43.030181.002425. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):372–395. doi: 10.1085/jgp.58.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., Ogden P. H. Transient changes in permeability in HeLa and L cells during detachment from a substrate. Q J Exp Physiol. 1987 Apr;72(2):189–199. doi: 10.1113/expphysiol.1987.sp003063. [DOI] [PubMed] [Google Scholar]
- Lambert I. H., Hoffmann E. K., Christensen P. Role of prostaglandins and leukotrienes in volume regulation by Ehrlich ascites tumor cells. J Membr Biol. 1987;98(3):247–256. doi: 10.1007/BF01871187. [DOI] [PubMed] [Google Scholar]
- Larson M., Spring K. R. Volume regulation by Necturus gallbladder: basolateral KCl exit. J Membr Biol. 1984;81(3):219–232. doi: 10.1007/BF01868715. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Hudson R. L., Schultz S. G. Cell swelling increases a barium-inhibitable potassium conductance in the basolateral membrane of Necturus small intestine. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3591–3594. doi: 10.1073/pnas.81.11.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. R., Hudson R. L., Schultz S. G. Effect of hypertonicity on the increase in basolateral conductance of Necturus small intestine in response to Na+-sugar cotransport. Biochim Biophys Acta. 1986 Feb 13;855(1):193–196. doi: 10.1016/0005-2736(86)90205-1. [DOI] [PubMed] [Google Scholar]
- Lauf P. K. K+:Cl- cotransport: sulfhydryls, divalent cations, and the mechanism of volume activation in a red cell. J Membr Biol. 1985;88(1):1–13. doi: 10.1007/BF01871208. [DOI] [PubMed] [Google Scholar]
- McGann L. E., Turner A. R., Turc J. M. Microcomputer interface for rapid measurements of average volume using an electronic particle counter. Med Biol Eng Comput. 1982 Jan;20(1):117–120. doi: 10.1007/BF02441862. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Tang J. M., Palmer L. G. Single-channel recordings of apical membrane chloride conductance in A6 epithelial cells. J Membr Biol. 1984;80(1):81–89. doi: 10.1007/BF01868692. [DOI] [PubMed] [Google Scholar]
- Okada Y. Solute transport process in intestinal epithelial cells. Membr Biochem. 1979;2(3-4):339–365. doi: 10.3109/09687687909063871. [DOI] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T., Yano J., Yawo H. Phagocytic activity and hyperpolarizing responses in L-strain mouse fibroblasts. J Physiol. 1981;313:101–119. doi: 10.1113/jphysiol.1981.sp013653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfrey H. C., Silva P., Epstein F. H. Sensitivity of cAMP-stimulated salt secretion in shark rectal gland to "loop" diuretics. Am J Physiol. 1984 Mar;246(3 Pt 1):C242–C246. doi: 10.1152/ajpcell.1984.246.3.C242. [DOI] [PubMed] [Google Scholar]
- Parker J. C. Hemolytic action of potassium salts on dog red blood cells. Am J Physiol. 1983 May;244(5):C313–C317. doi: 10.1152/ajpcell.1983.244.5.C313. [DOI] [PubMed] [Google Scholar]
- Reuss L. Changes in cell volume measured with an electrophysiologic technique. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6014–6018. doi: 10.1073/pnas.82.17.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti L. W., Rothstein A. Adaptation of mouse leukemic cells (L5178Y) to anisotonic media. I. Cell volume regulation. Exp Cell Res. 1973 Jun;79(2):295–310. doi: 10.1016/0014-4827(73)90448-5. [DOI] [PubMed] [Google Scholar]
- Roy G., Sauvé R. Effect of anisotonic media on volume, ion and amino-acid content and membrane potential of kidney cells (MDCK) in culture. J Membr Biol. 1987;100(1):83–96. doi: 10.1007/BF02209143. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Cheung R., Mack E., Grinstein S., Gelfand E. W., Rothstein A. Cation and anion transport pathways in volume regulatory response of human lymphocytes to hyposmotic media. Am J Physiol. 1985 May;248(5 Pt 1):C480–C487. doi: 10.1152/ajpcell.1985.248.5.C480. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Mack E., Rothstein A. Ionic events during the volume response of human peripheral blood lymphocytes to hypotonic media. I. Distinctions between volume-activated Cl- and K+ conductance pathways. J Gen Physiol. 1984 Apr;83(4):497–512. doi: 10.1085/jgp.83.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. I. Kinetics of cation transport under hypertonic conditions. J Gen Physiol. 1977 Jul;70(1):59–79. doi: 10.1085/jgp.70.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Fuisz R. E., Curran P. F. Amino acid and sugar transport in rabbit ileum. J Gen Physiol. 1966 May;49(5):849–866. doi: 10.1085/jgp.49.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda F. V., Burton K. A., Brown P. D. Calcium movements accompanying the transport of sugar or amino acid by rabbit enterocytes. Biochim Biophys Acta. 1986 Mar 27;856(1):185–187. doi: 10.1016/0005-2736(86)90026-x. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Ericson A. C. Epithelial cell volume modulation and regulation. J Membr Biol. 1982;69(3):167–176. doi: 10.1007/BF01870396. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- White M. M., Miller C. A voltage-gated anion channel from the electric organ of Torpedo californica. J Biol Chem. 1979 Oct 25;254(20):10161–10166. [PubMed] [Google Scholar]
- Wills N. K. Apical membrane potassium and chloride permeabilities in surface cells of rabbit descending colon epithelium. J Physiol. 1985 Jan;358:433–445. doi: 10.1113/jphysiol.1985.sp015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T., Oiki S., Ueda S., Okada Y. Synchronous oscillation of the cytoplasmic Ca2+ concentration and membrane potential in cultured epithelial cells (Intestine 407). Biochim Biophys Acta. 1986 Jun 16;887(1):105–112. doi: 10.1016/0167-4889(86)90129-1. [DOI] [PubMed] [Google Scholar]
- Yada T., Okada Y. Electrical activity of an intestinal epithelial cell line: hyperpolarizing responses to intestinal secretagogues. J Membr Biol. 1984;77(1):33–44. doi: 10.1007/BF01871098. [DOI] [PubMed] [Google Scholar]


