Abstract
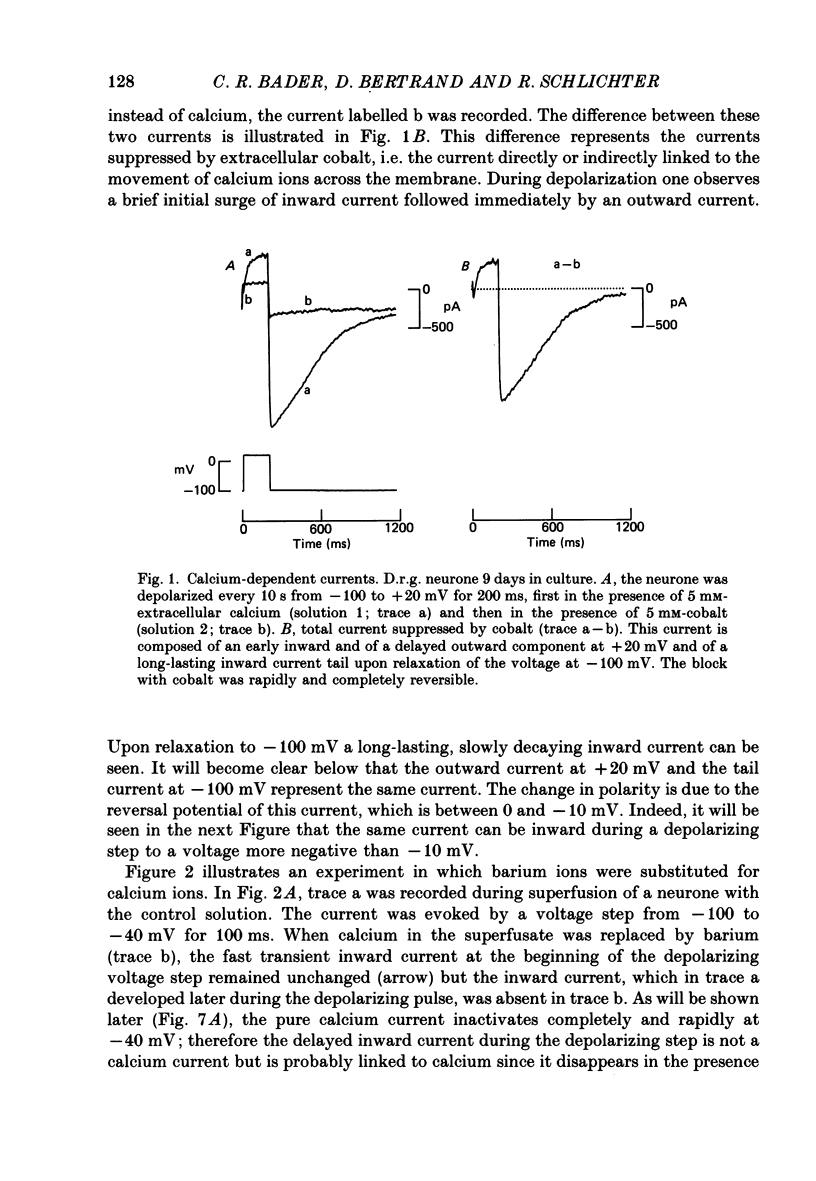
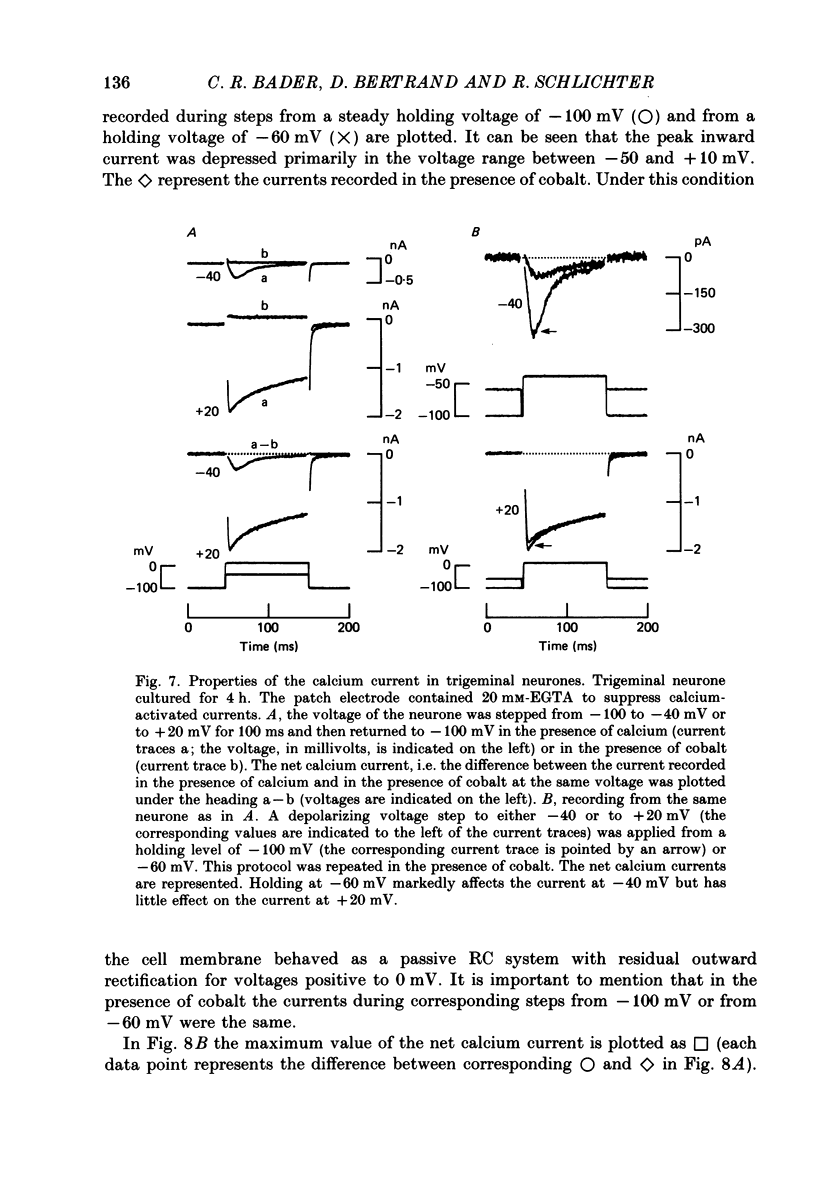
1. Sensory (trigeminal and dorsal root) and autonomic (ciliary) ganglia from embryonic quail were dissociated and the neurones were grown in tissue culture. 2. Intracellular recordings were made in voltage clamp using patch electrodes and the whole-cell recording technique. In order to investigate a calcium-activated chloride current, the sodium and potassium currents were blocked. 3. Depolarizing voltage steps from a holding potential of -100 mV to a test potential of +20 mV triggered an early inward and a delayed outward current. The latter persisted as a long-lasting inward tail current when the membrane was depolarized to -100 mV. 4. These currents were all blocked by extracellular cobalt suggesting that they were calcium dependent. During a test depolarization to +20 mV, in the presence of intracellular EGTA (20 mM), the inward current persisted but the outward current was suppressed. EGTA (20 mM) also suppressed the long-lasting inward tail current at -100 mV. This suggested the presence of a calcium-activated current. 5. The reversal potential of the calcium-activated current was near the equilibrium potential for chloride ions and was shifted as predicted by the Nernst equation when the extracellular chloride concentration was changed. 6. The calcium-activated current was partially blocked by adding 4-acetamido-4'-isothiocyanatostilbene-disulphonic acid (SITS) at a concentration of 1 mM to the external superfusion medium. This effect of a compound known to interfere with chloride channels together with the results of point (5) suggested the existence of a calcium-activated chloride current (ICl(Ca)). 7. ICl(Ca) could be activated by transient and sustained components of the calcium current present in the cultured neurones. 8. ICl(Ca) was present in 80% of the sensory neurones but only in 10% of the parasympathetic neurones.
Full text
PDF






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D., Dupin E., Kato A. C. Development of electrical membrane properties in cultured avian neural crest. 1983 Oct 27-Nov 2Nature. 305(5937):808–810. doi: 10.1038/305808a0. [DOI] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Dupin E. Voltage-dependent potassium currents in developing neurones from quail mesencephalic neural crest. J Physiol. 1985 Sep;366:129–151. doi: 10.1113/jphysiol.1985.sp015789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Kato A. C. Chick ciliary ganglion in dissociated cell culture. II. Electrophysiological properties. Dev Biol. 1982 Nov;94(1):131–141. doi: 10.1016/0012-1606(82)90076-8. [DOI] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D., Bader C. R. DATAC: a multipurpose biological data analysis program based on a mathematical interpreter. Int J Biomed Comput. 1986 May;18(3-4):193–202. doi: 10.1016/0020-7101(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Cand P., Henauer R., Bader C. R. Fabrication of glass microelectrodes with microprocessor control. J Neurosci Methods. 1983 Feb;7(2):171–183. doi: 10.1016/0165-0270(83)90080-8. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M., Feltz P., Lamour Y. A model for an estimate in vivo of the ionic basis of presynaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res. 1976 Dec 24;118(3):486–493. doi: 10.1016/0006-8993(76)90318-8. [DOI] [PubMed] [Google Scholar]
- Dodd J., Jahr C. E., Hamilton P. N., Heath M. J., Matthew W. D., Jessell T. M. Cytochemical and physiological properties of sensory and dorsal horn neurons that transmit cutaneous sensation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):685–695. doi: 10.1101/sqb.1983.048.01.072. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A., Tan Y. P., Trautmann A. Blockage of Ca-activated Cl conductance by furosemide in rat lacrimal glands. Pflugers Arch. 1986 Jan;406(1):65–68. doi: 10.1007/BF00582955. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Nakamura J., Shinnick-Gallagher P. The effects of temperature, pH and Cl-pump inhibitors on GABA responses recorded from cat dorsal root ganglia. Brain Res. 1983 May 16;267(2):249–259. doi: 10.1016/0006-8993(83)90877-6. [DOI] [PubMed] [Google Scholar]
- Gray P. T., Ritchie J. M. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985 Apr;85(4):519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A. C., Rey M. J. Chick ciliary ganglion in dissociated cell culture. I. Cholinergic properties. Dev Biol. 1982 Nov;94(1):121–130. doi: 10.1016/0012-1606(82)90075-6. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. Cell line segregation during peripheral nervous system ontogeny. Science. 1986 Mar 28;231(4745):1515–1522. doi: 10.1126/science.3952494. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern A. E., Margiotta J. F., Berg D. K. gamma-Aminobutyric acid receptors on chick ciliary ganglion neurons in vivo and in cell culture. J Neurosci. 1985 Oct;5(10):2690–2695. doi: 10.1523/JNEUROSCI.05-10-02690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. Voltage-clamp analysis of a Ca2+- and voltage-dependent chloride conductance in cultured mouse spinal neurons. J Neurophysiol. 1986 Jun;55(6):1115–1135. doi: 10.1152/jn.1986.55.6.1115. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Clermont Y., Beaudet A. Ultrastructural features of six types of neurons in rat dorsal root ganglia. J Neurocytol. 1983 Feb;12(1):47–66. doi: 10.1007/BF01148087. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Brodwick M. S. Properties of chloride transport in barnacle muscle fibers. J Gen Physiol. 1979 Mar;73(3):343–368. doi: 10.1085/jgp.73.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziller C., Dupin E., Brazeau P., Paulin D., Le Douarin N. M. Early segregation of a neuronal precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell. 1983 Feb;32(2):627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]



