Abstract
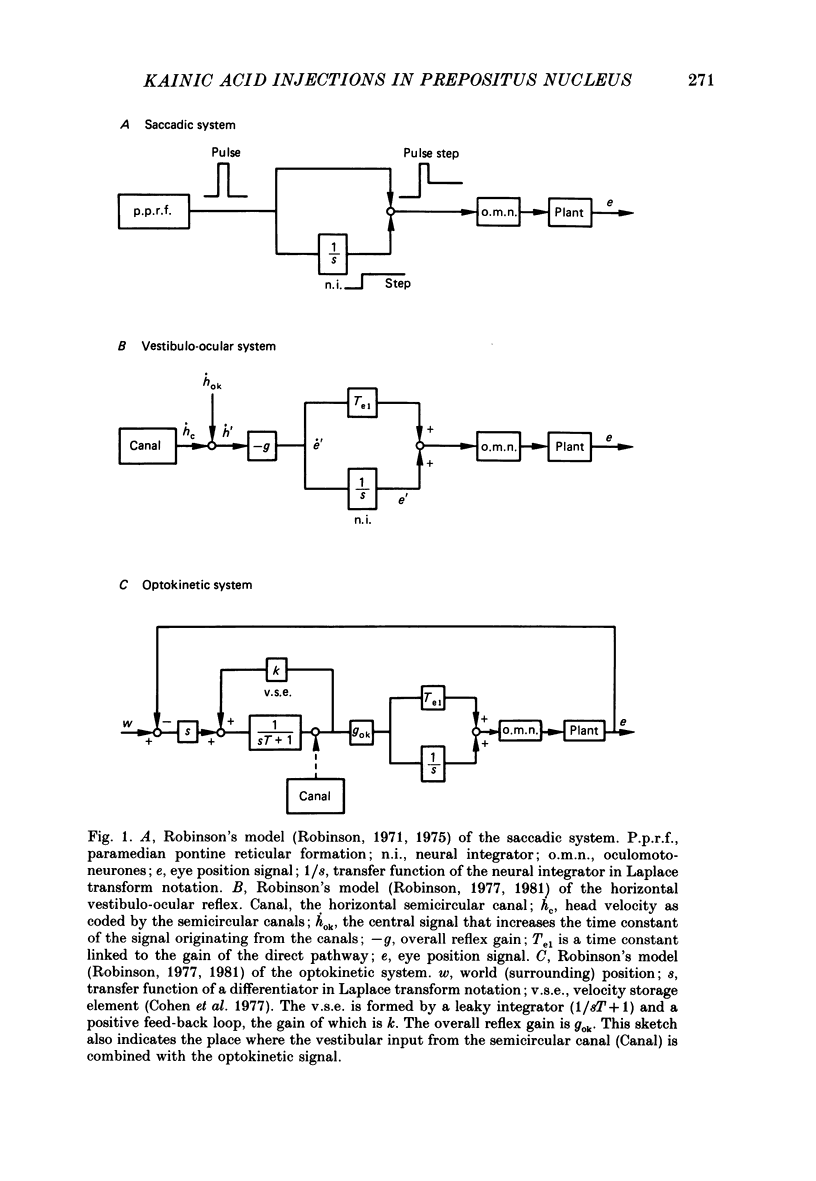
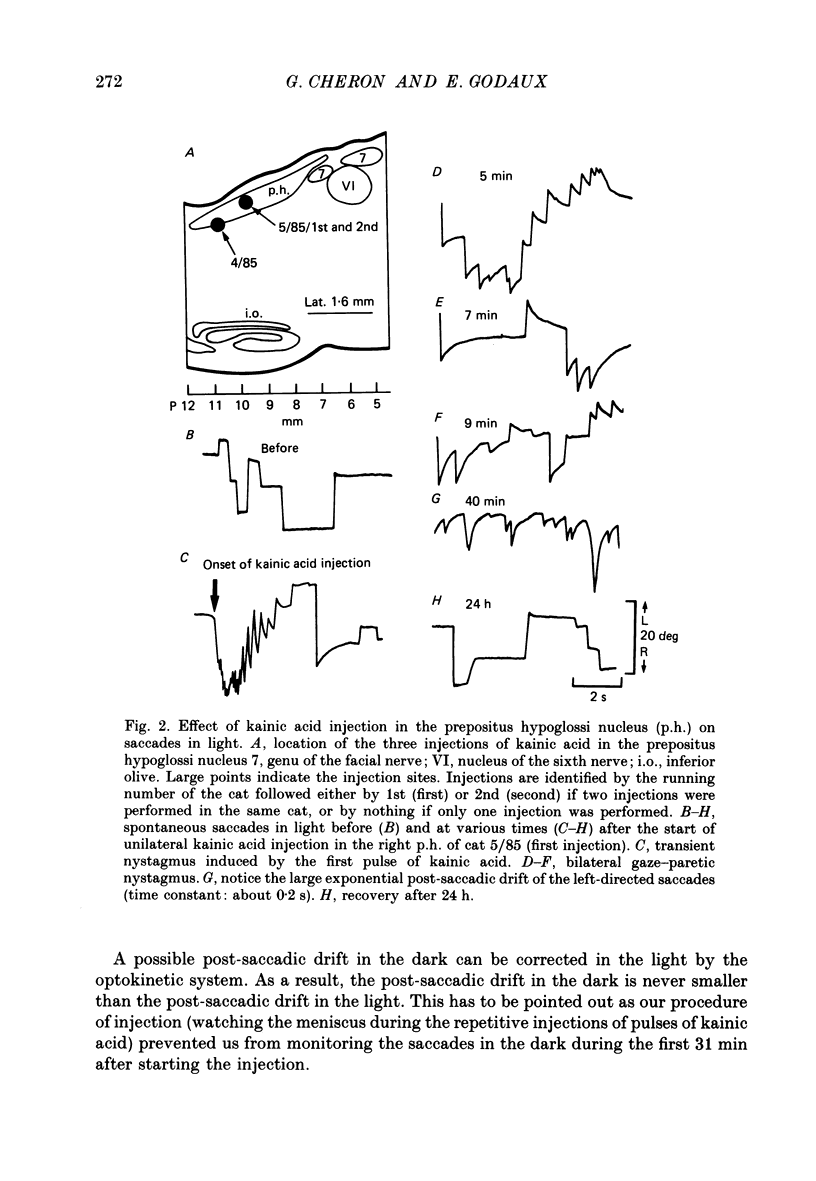
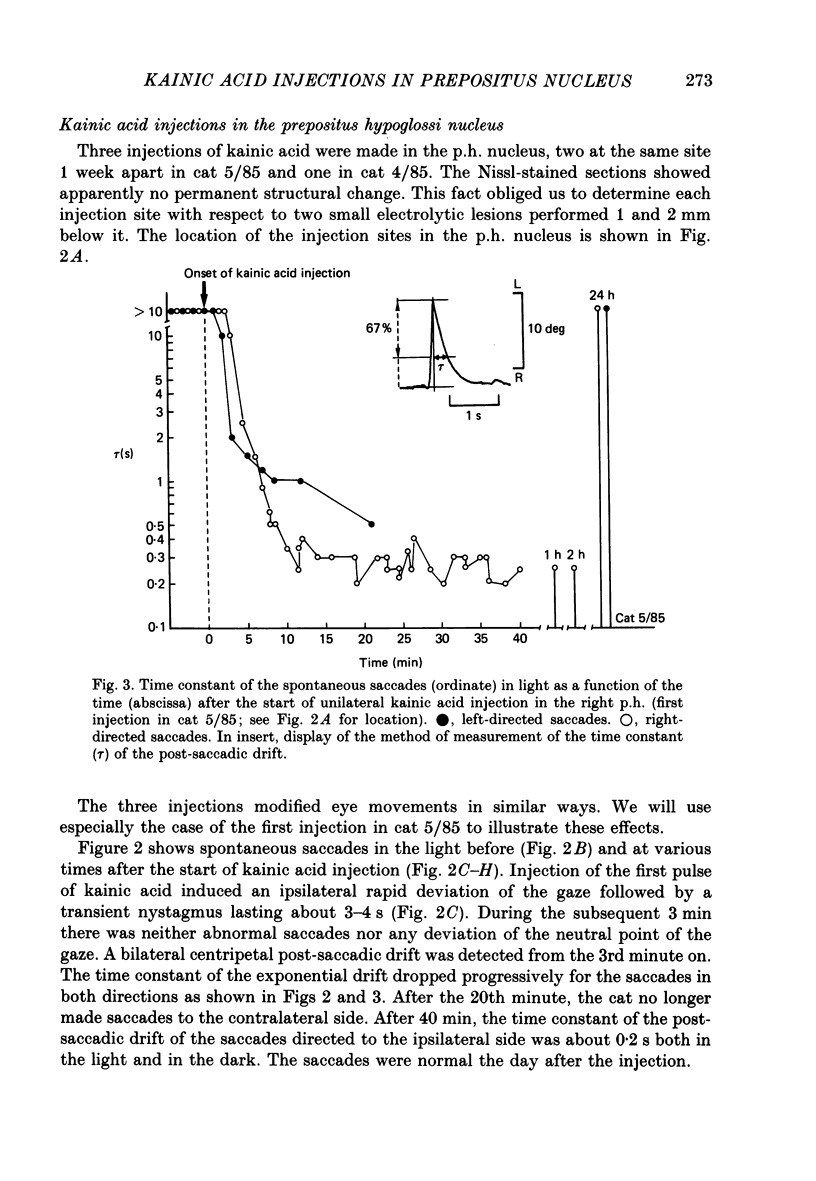
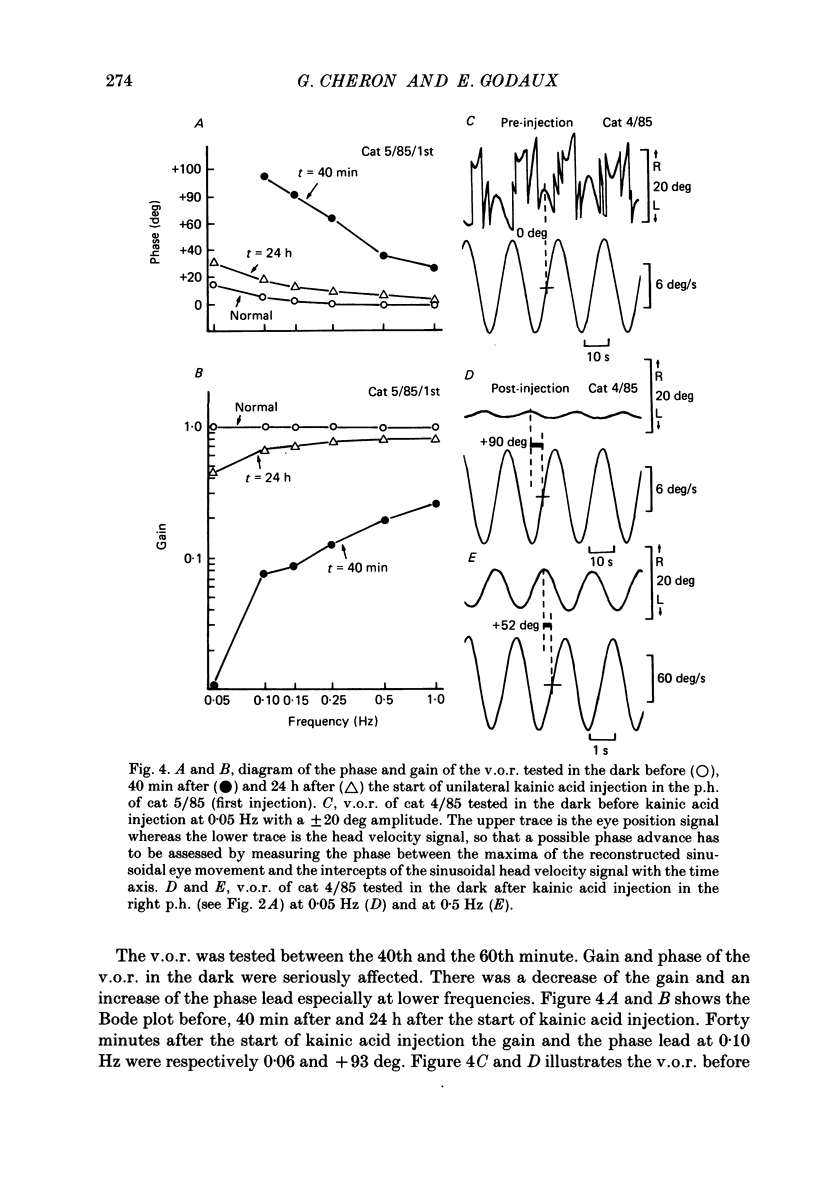
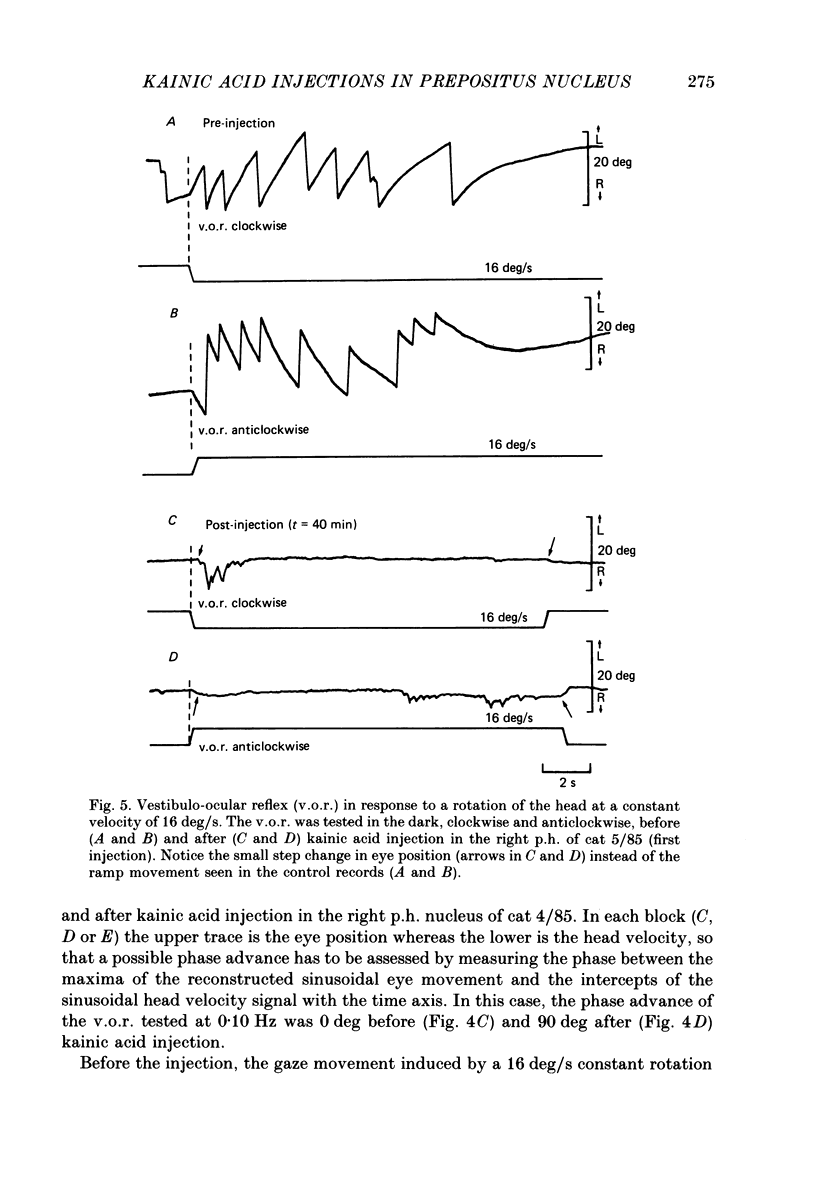
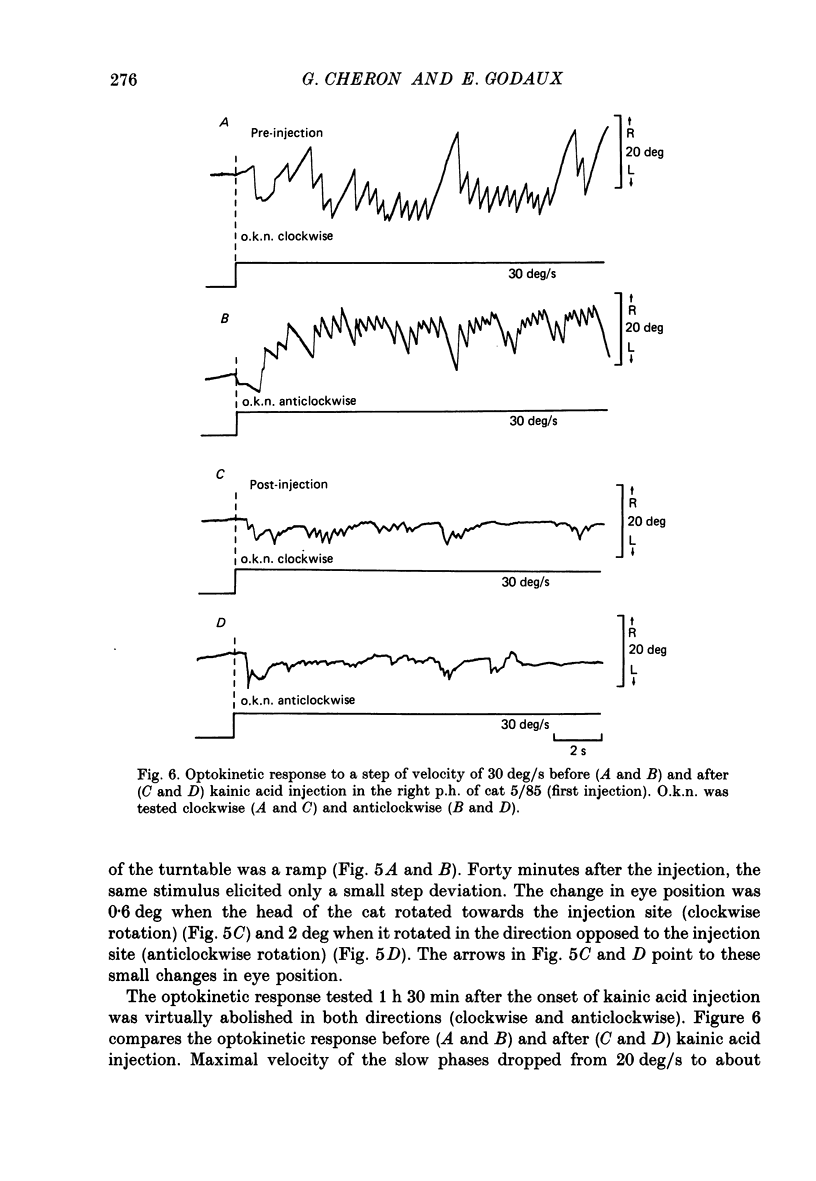
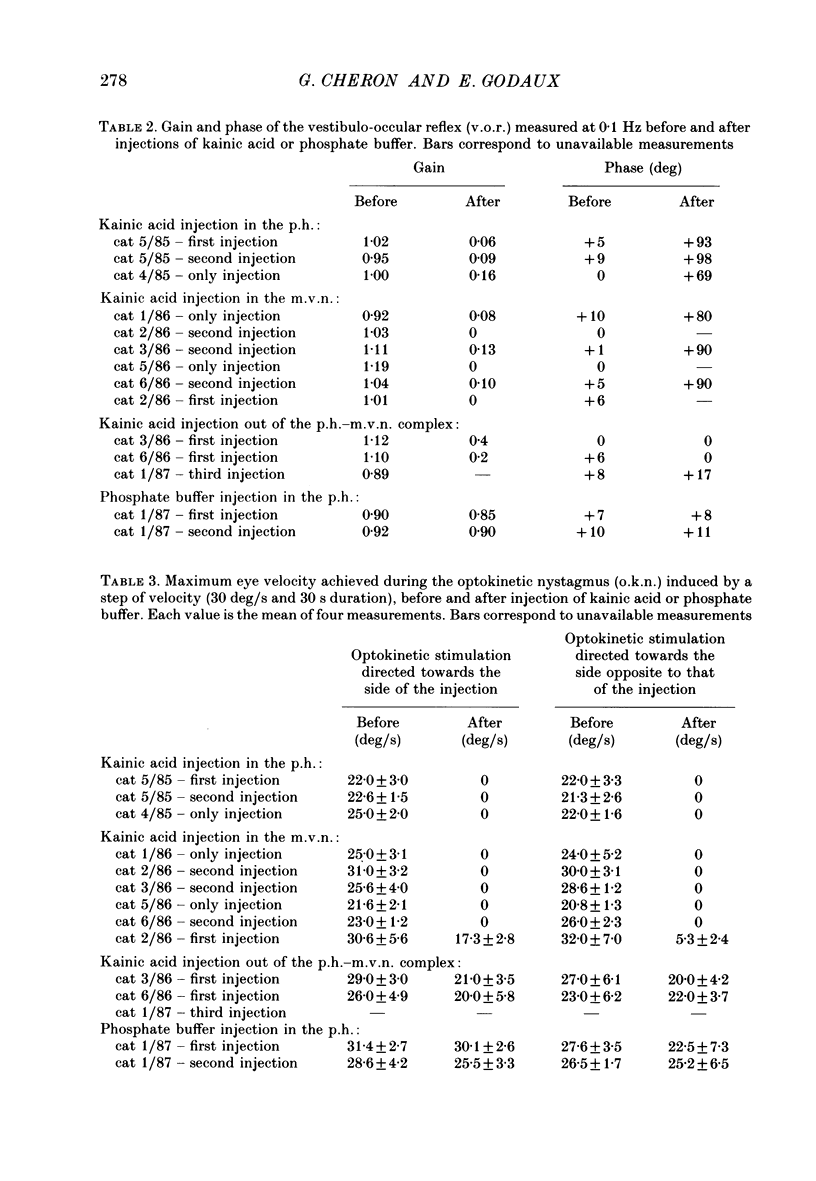
1. This study was intended to test the candidature of the prepositus-vestibular nuclear complex for being the location of the oculomotor neural integrator (Robinson's integrator). 2. Microinjections of kainic acid (2 micrograms dissolved in 1 microliter) were made in awake cats. Injection sites were located either in the prepositus hypoglossi nucleus (p.h.), the medial vestibular nucleus (m.v.n.), the medial longitudinal fasciculus (m.l.f.) or in the magnocellular tegmental field of the reticular formation. 3. Theory predicts that a complete disabling of the neural integrator will cause (a) an exponential post-saccadic drift whose time constant will be 0.16 s in the dark (b) a phase lead of +93 deg as the vestibulo-ocular reflex is tested at 0.10 Hz in the dark and (c) a nearly complete abolition of the optokinetic nystagmus (o.k.n.). 4. About 1 h after a unilateral kainic acid injection in the p.h., we observed (a) a large bilateral post-saccadic drift (time constant sometimes as low as 0.2 s) (b) a large phase lead at 0.10 Hz (range: from +69 to +98 deg) (c) an abolition of the o.k.n. control injection of phosphate buffer in the p.h. did not produce any deficit. 5. A unilateral kainic acid injection in the m.v.n. induced a nystagmus followed by signs of bilateral failure of the neural integrator similar to those observed after kainic acid injection in the p.h. 6. Injection near the mid-line, between the two p.h. nuclei, induced a defect of the neural integrator less than that observed after kainic acid injection in either the p.h. or the m.v.n. Injection of kainic acid in the magnocellular tegmental field of the reticular formation did not produce any sign of failure of the neural integrator. No post-saccadic drift was observed. 7. We have concluded that (a) the p.h. nucleus is involved in the integration processing, and that (b) the m.v.n. is involved either in the integration processing or in the relaying of the output of the neural integrator to the oculomotoneurones.
Full text
PDF



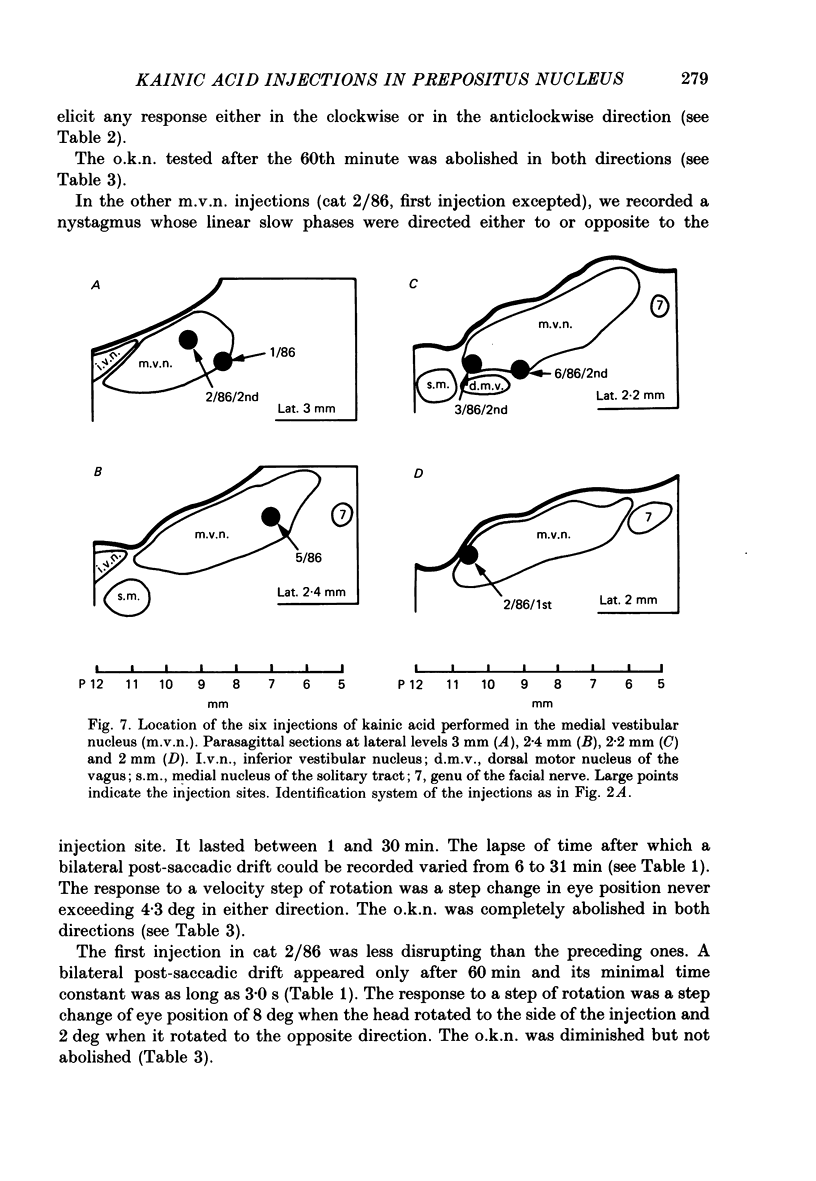
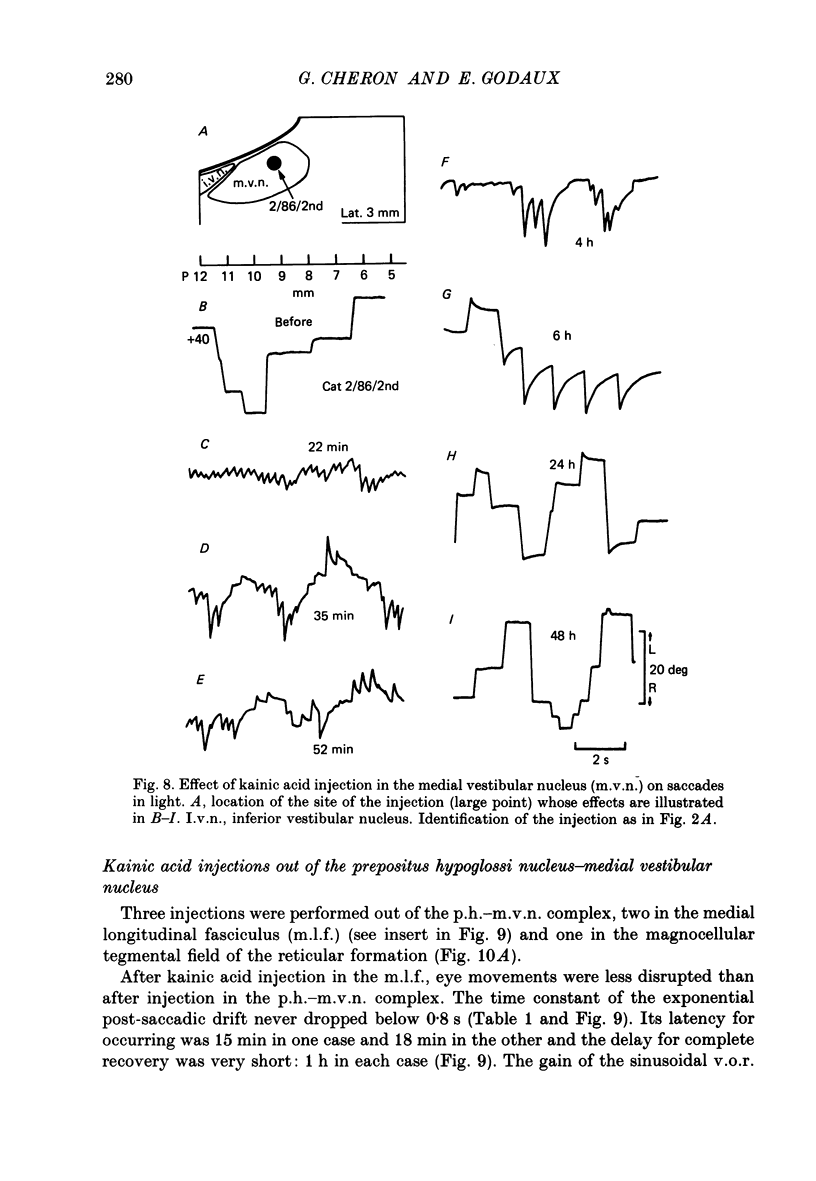
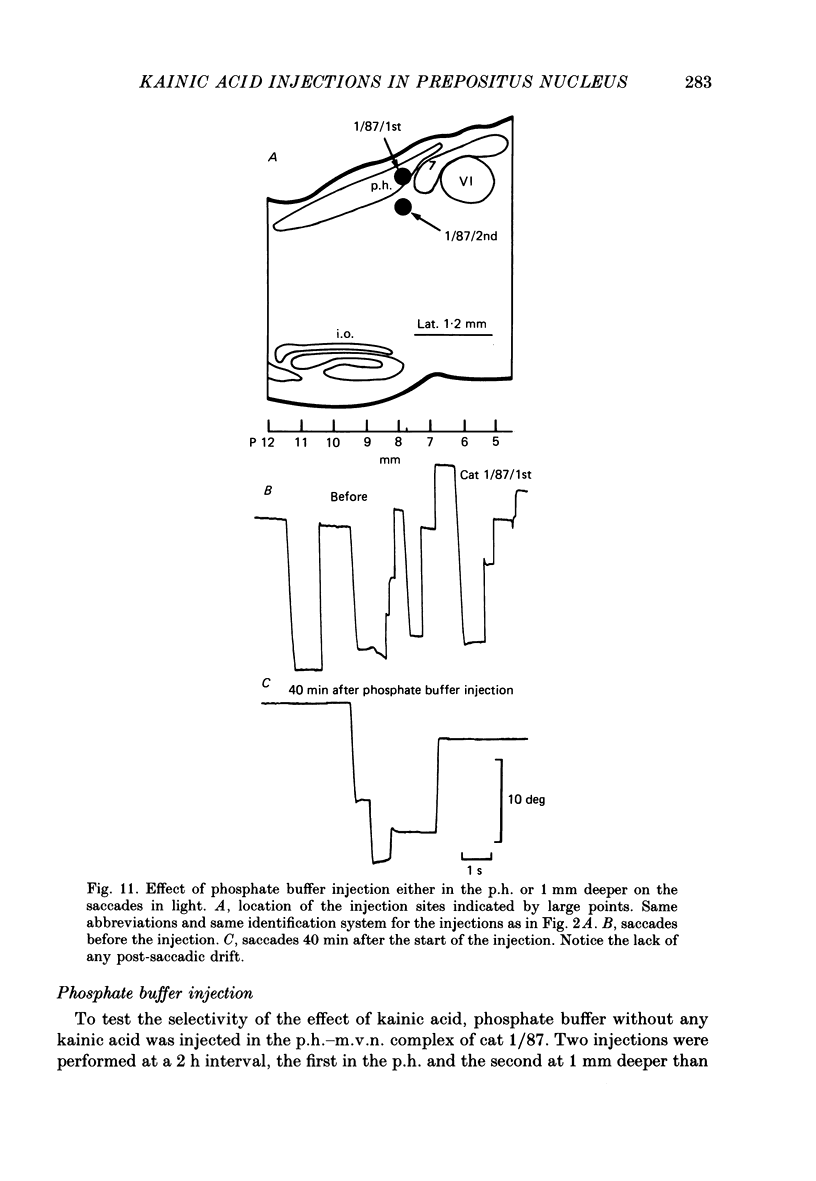
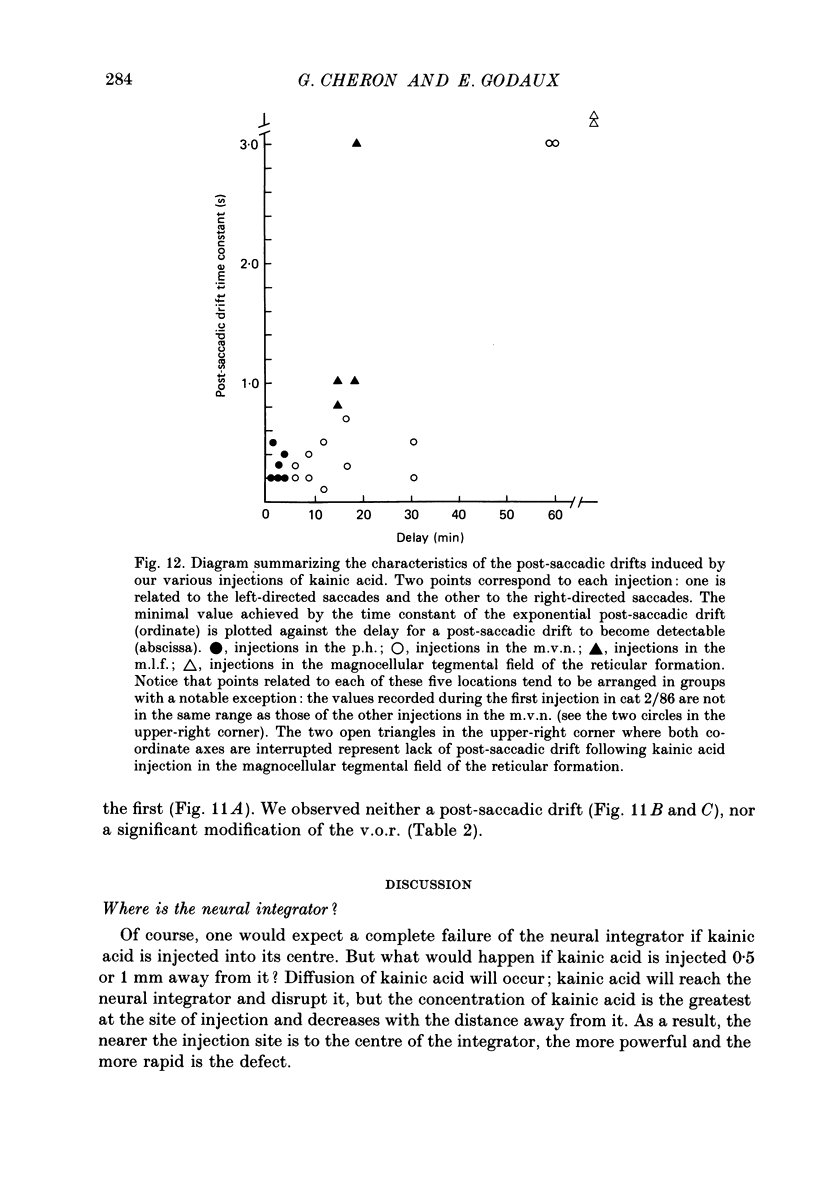






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
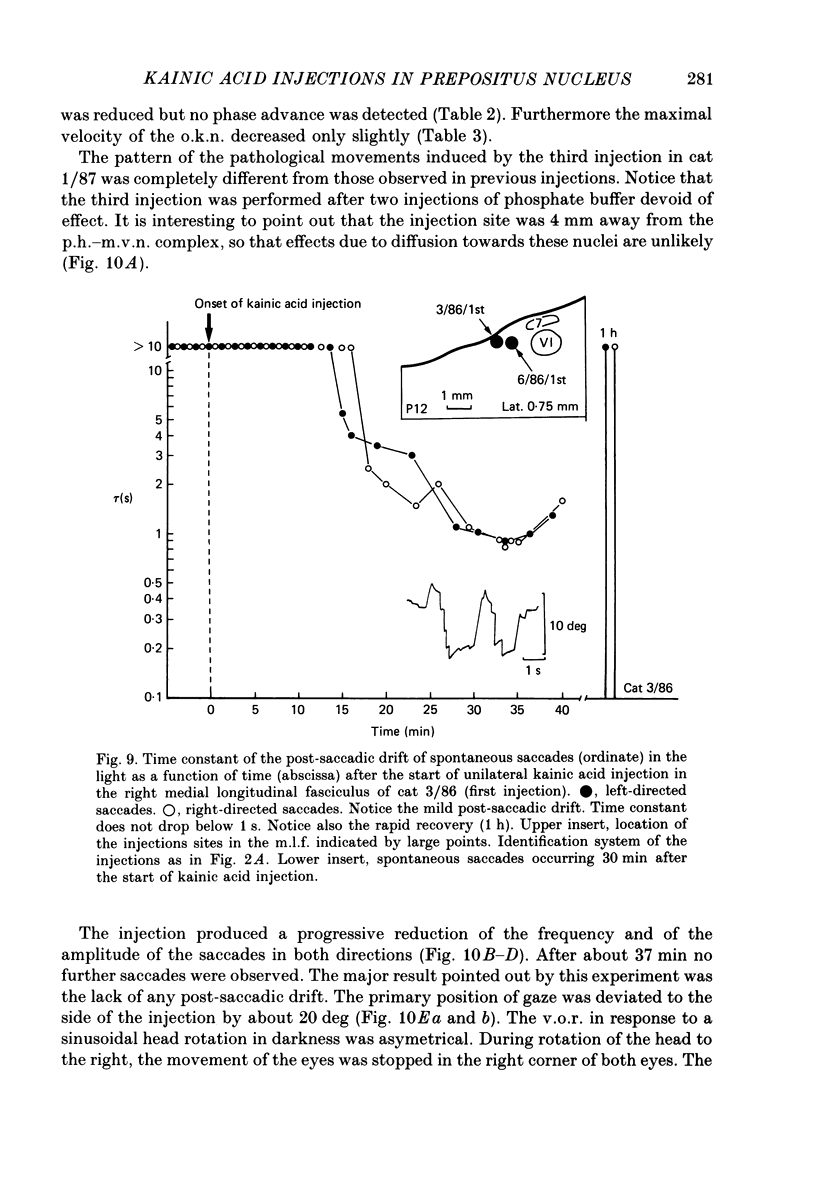
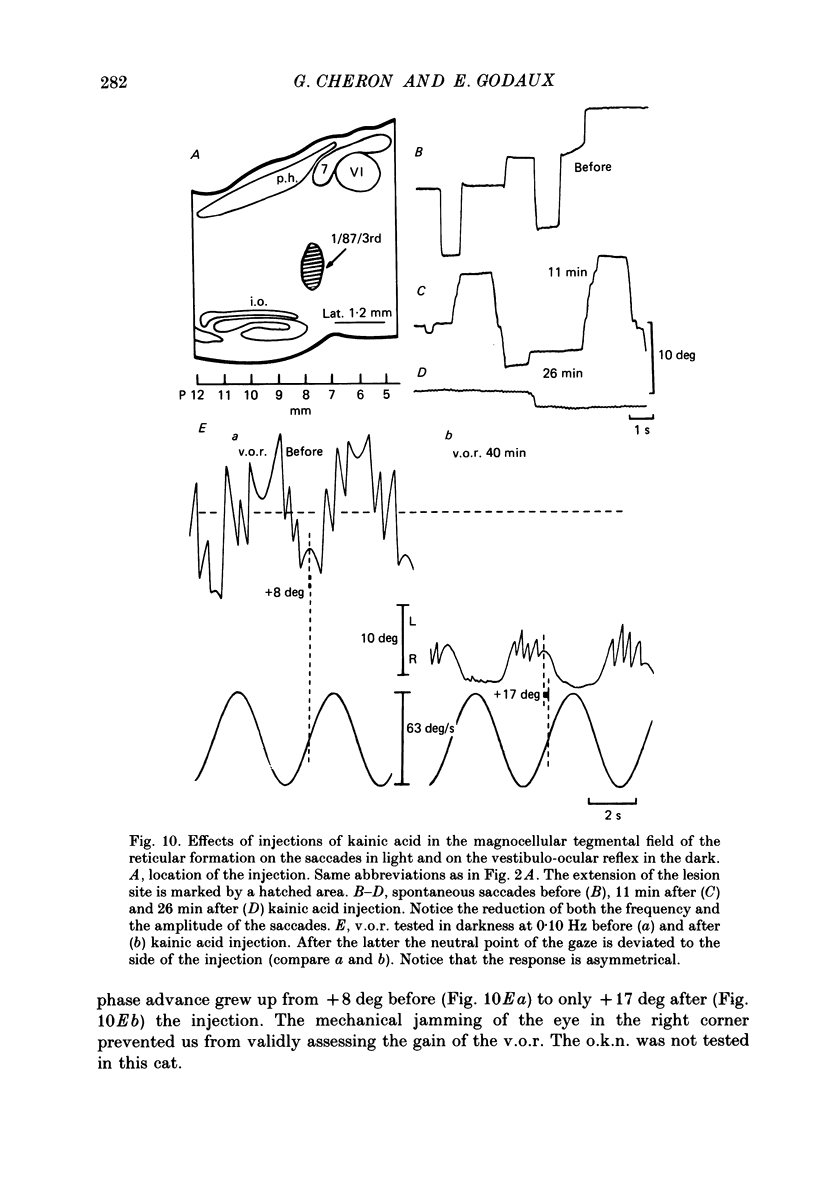
- Albano J. E., Wurtz R. H. Deficits in eye position following ablation of monkey superior colliculus, pretectum, and posterior-medial thalamus. J Neurophysiol. 1982 Aug;48(2):318–337. doi: 10.1152/jn.1982.48.2.318. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Robinson D. A., Shamma S. A proposed neural network for the integrator of the oculomotor system. Biol Cybern. 1983;49(2):127–136. doi: 10.1007/BF00320393. [DOI] [PubMed] [Google Scholar]
- Cheron G., Gillis P., Godaux E. Lesions in the cat prepositus complex: effects on the optokinetic system. J Physiol. 1986 Mar;372:95–111. doi: 10.1113/jphysiol.1986.sp015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G., Godaux E., Laune J. M., Vanderkelen B. Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. J Physiol. 1986 Mar;372:75–94. doi: 10.1113/jphysiol.1986.sp015998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol. 1972 Jul;36(1):101–117. doi: 10.1016/0014-4886(72)90139-2. [DOI] [PubMed] [Google Scholar]
- Cohen B., Matsuo V., Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977 Sep;270(2):321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Molliver M. E., Kuhar M. J. In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J Comp Neurol. 1978 Jul 15;180(2):301–323. doi: 10.1002/cne.901800208. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Robinson D. A. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966 May;21(3):1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Galiana H. L., Outerbridge J. S. A bilateral model for central neural pathways in vestibuloocular reflex. J Neurophysiol. 1984 Feb;51(2):210–241. doi: 10.1152/jn.1984.51.2.210. [DOI] [PubMed] [Google Scholar]
- Godaux E., Laune J. M. The saccadic system and the vestibulo-ocular reflex in the cat do not share the same integrator. Neurosci Lett. 1983 Aug 8;38(3):263–268. doi: 10.1016/0304-3940(83)90379-8. [DOI] [PubMed] [Google Scholar]
- Henn V., Lang W., Hepp K., Reisine H. Experimental gaze palsies in monkeys and their relation to human pathology. Brain. 1984 Jun;107(Pt 2):619–636. doi: 10.1093/brain/107.2.619. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Wurtz R. H. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985 Jan;53(1):266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Judge S. J., Richmond B. J., Chu F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Keller E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974 Mar;37(2):316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J., Darlot C., Berthoz A., Baker R. Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. J Neurophysiol. 1982 Feb;47(2):329–352. doi: 10.1152/jn.1982.47.2.329. [DOI] [PubMed] [Google Scholar]
- Magnin M., Salinger W., Kennedy H. Optokinetic response and visual suppression of the vestibulo-ocular reflex in the open loop condition in the cat. Vision Res. 1986;26(4):653–660. doi: 10.1016/0042-6989(86)90013-1. [DOI] [PubMed] [Google Scholar]
- McCrea R. A., Baker R. Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol. 1985 Jul 15;237(3):377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- Pola J., Robinson D. A. Oculomotor signals in medial longitudinal fasciculus of the monkey. J Neurophysiol. 1978 Mar;41(2):245–259. doi: 10.1152/jn.1978.41.2.245. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976 Sep;39(5):954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. The effect of cerebellectomy on the cat's bestibulo-ocular integrator. Brain Res. 1974 May 17;71(2-3):195–207. doi: 10.1016/0006-8993(74)90961-5. [DOI] [PubMed] [Google Scholar]
- Shimazu H., Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol. 1966 May;29(3):467–492. doi: 10.1152/jn.1966.29.3.467. [DOI] [PubMed] [Google Scholar]
- Skavenski A. A., Robinson D. A. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973 Jul;36(4):724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H., Albano J. E. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci. 1980;3:189–226. doi: 10.1146/annurev.ne.03.030180.001201. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H., Goldberg M. E. Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movements. J Neurophysiol. 1972 Jul;35(4):587–596. doi: 10.1152/jn.1972.35.4.587. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yamazaki A., Butler P. H., Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981 Oct;46(4):878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]


