Abstract
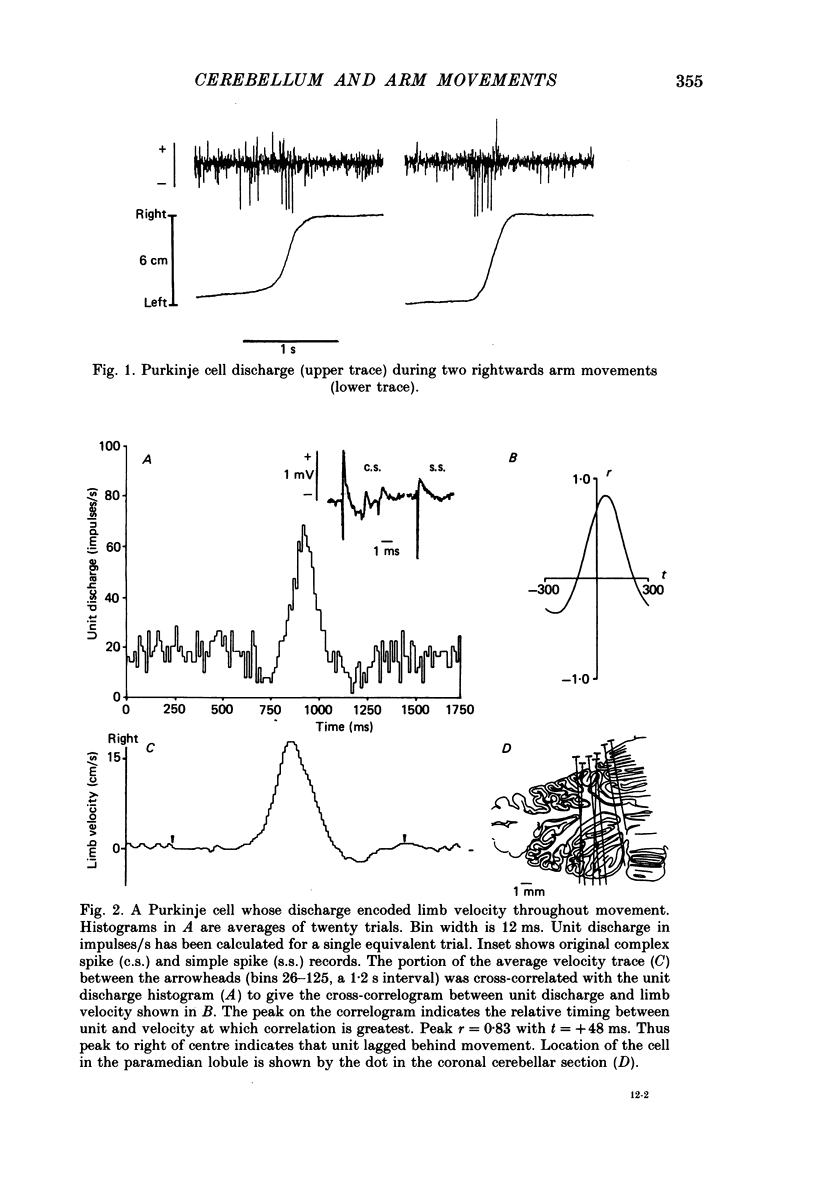
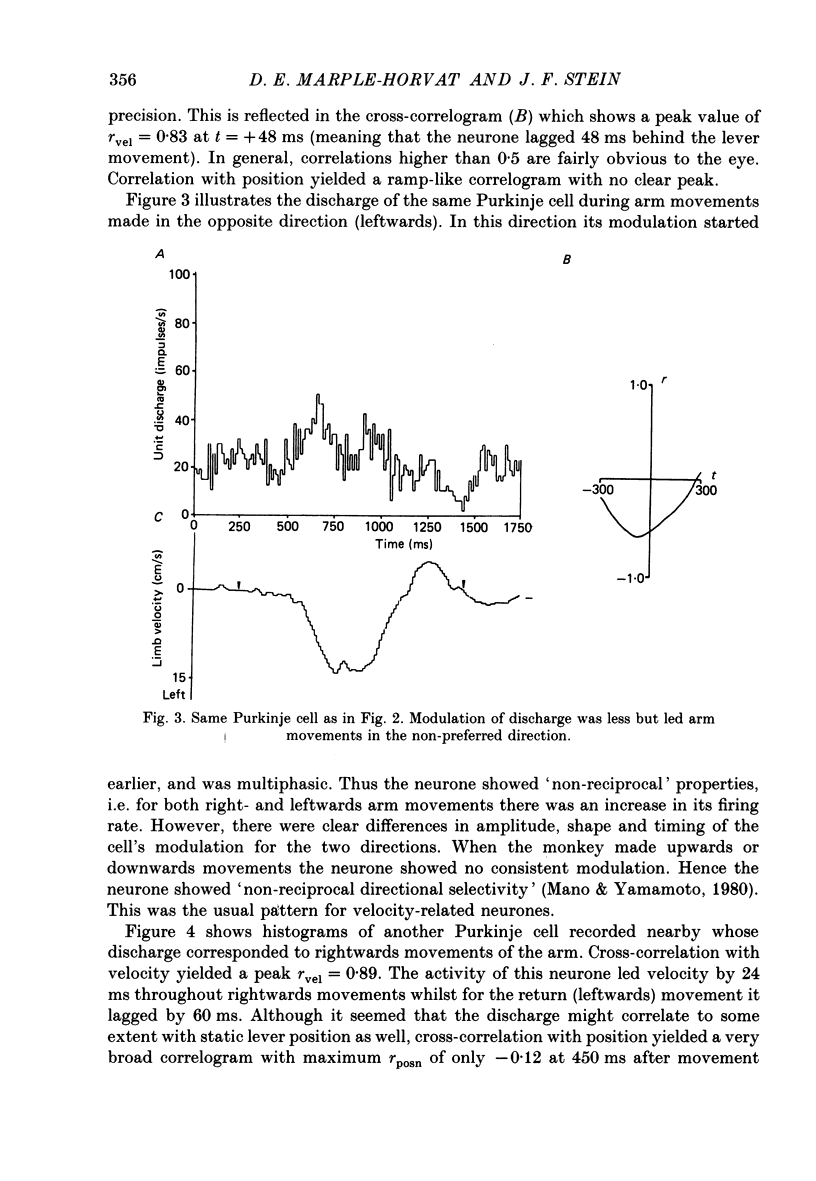
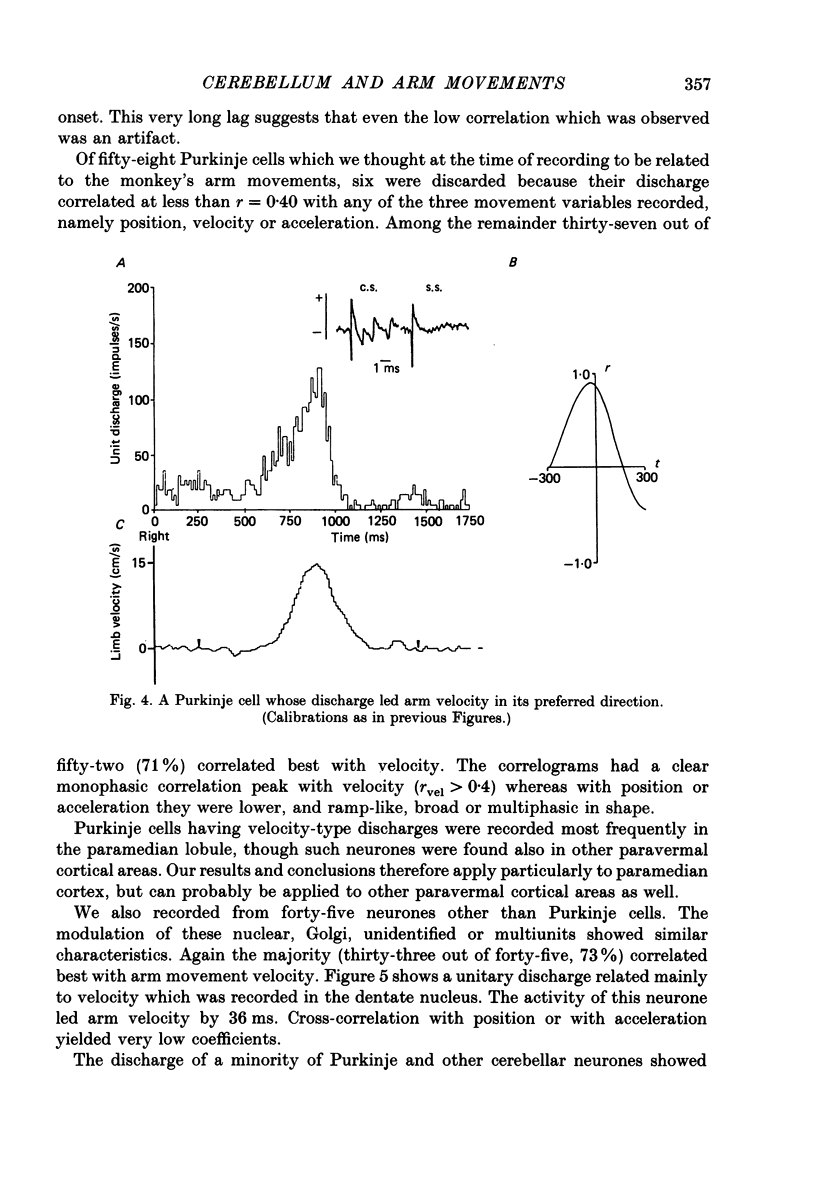
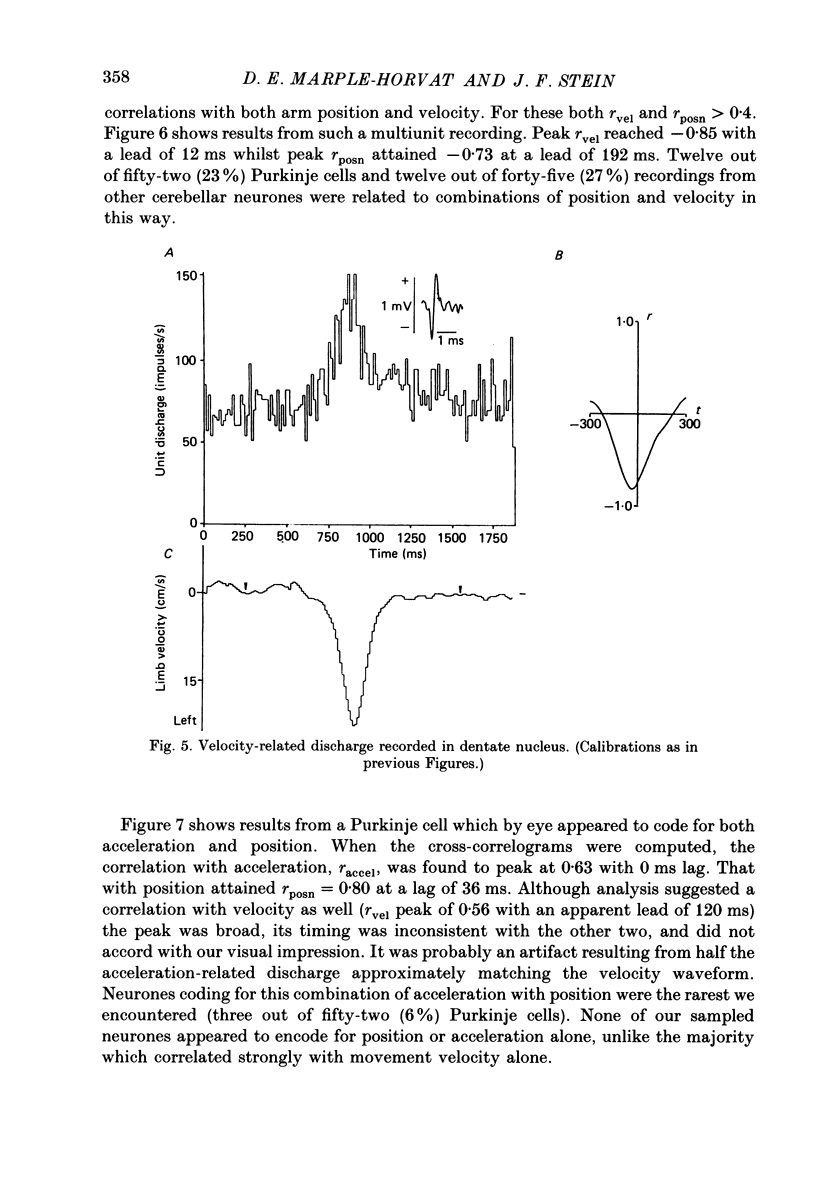
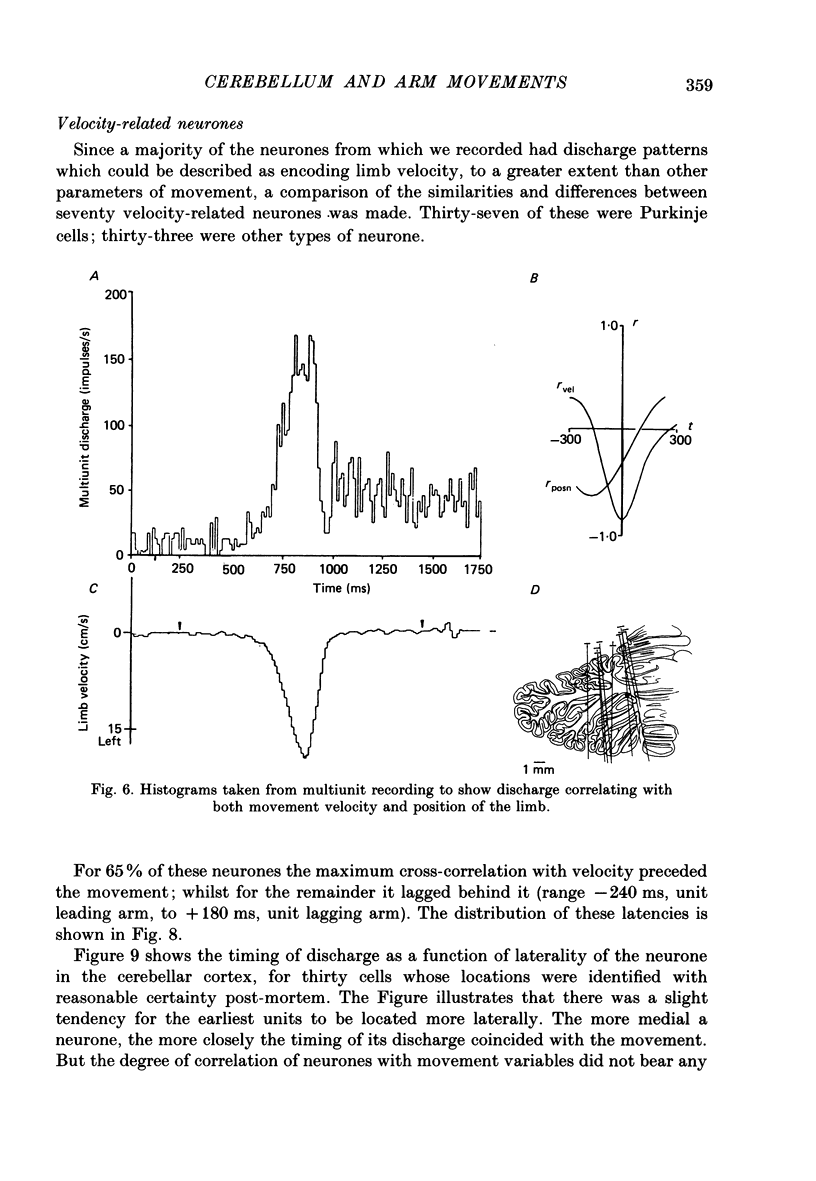
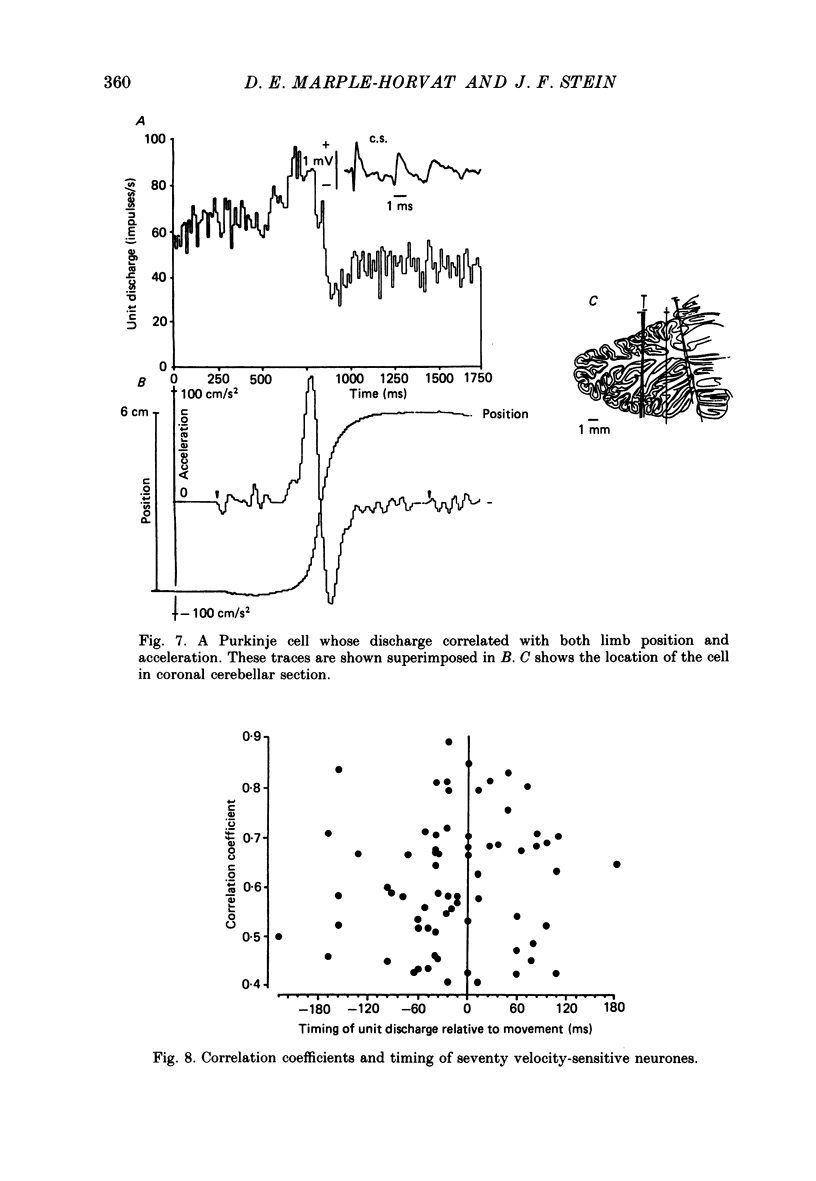
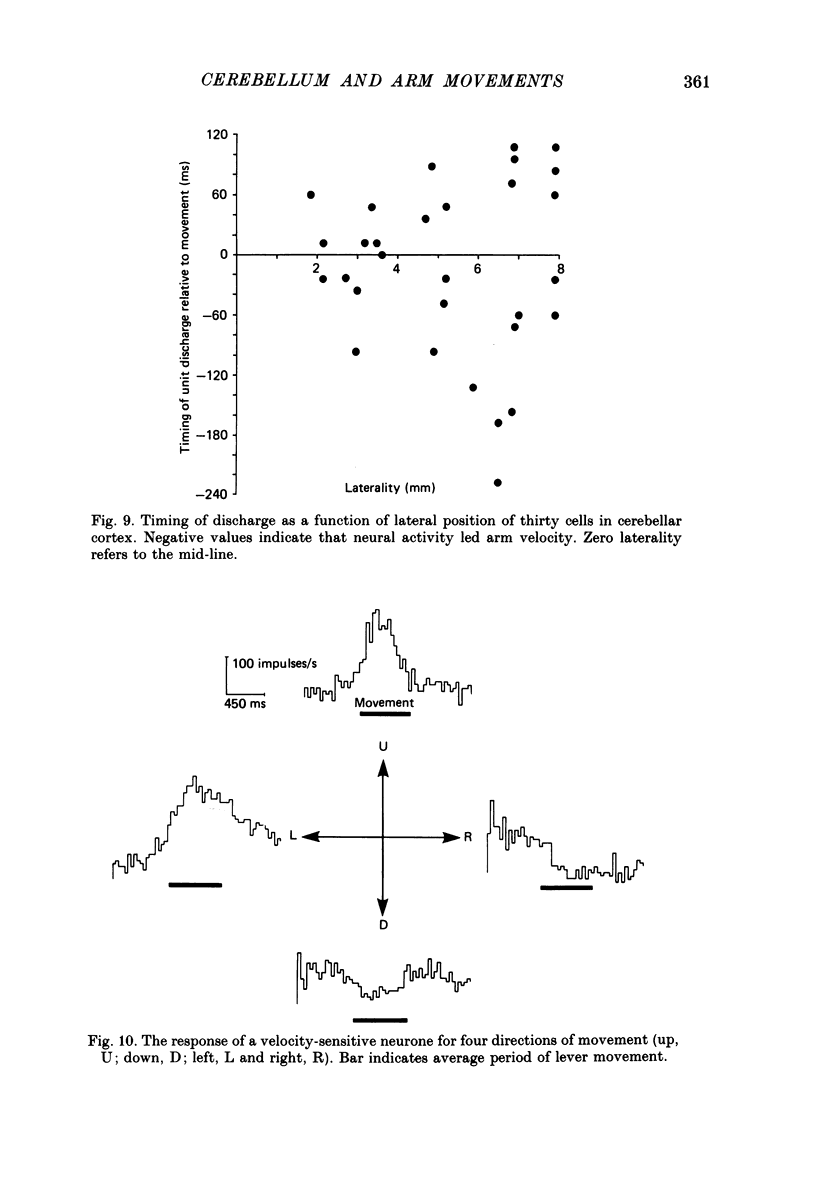
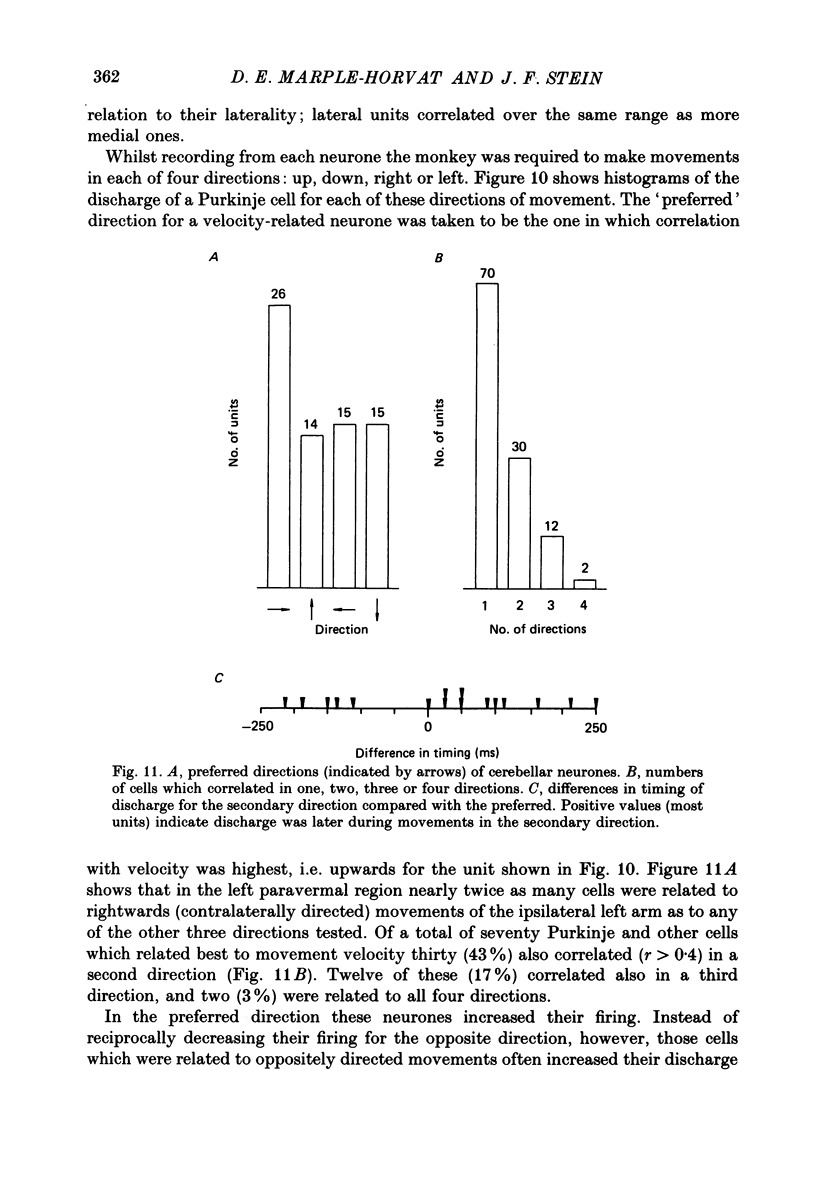
1. The role of the paravermal cerebellum in controlling arm movements in monkeys trained to perform visually guided movements was investigated. Discharge patterns of extracellularly recorded Purkinje, Golgi, nuclear and unidentified cells were correlated with arm position, velocity and acceleration. 2. The discharge of thirty-seven out of fifty-two movement-related Purkinje cells and that of thirty-three out of forty-five other movement-related cerebellar neurones was more highly correlated with limb velocity (0.95 greater than r greater than 0.4) than with position or acceleration. Twelve out of fifty-two Purkinje cells and twelve out of forty-five other cells were related to both limb velocity and limb position. Three Purkinje cells were related to limb acceleration and position and no cell was related to position or acceleration alone. 3. Modulation of discharge of these cells usually preceded movement (range -240 ms, unit leading movement, to +180 ms, unit lagging; mode -36 ms; mean -15 ms). 4. Each movement-related neurone was tested for four directions of movement. All showed a preferred direction in which the correlation with arm movement velocity was highest. However, 43% (30/70) correlated at r greater than 0.4 with movements in a second direction, usually that opposite to their preferred direction. Sixty-three per cent of these neurones fired earlier during movements in the preferred than in the opposite direction. 5. It is concluded that the paravermal cerebellum may be involved in computing the velocity vectors required for achieving properly directed and co-ordinated movements of the whole arm.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J., Gibson A., Glickstein M., Stein J. Visual cells in the pontine nuclei of the cat. J Physiol. 1976 Feb;255(2):415–433. doi: 10.1113/jphysiol.1976.sp011287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biguer B., Prablanc C., Jeannerod M. The contribution of coordinated eye and head movements in hand pointing accuracy. Exp Brain Res. 1984;55(3):462–469. doi: 10.1007/BF00235277. [DOI] [PubMed] [Google Scholar]
- Bizzi E., Kalil R. E., Tagliasco V. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science. 1971 Jul 30;173(3995):452–454. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol. 1977 Jan;264(3):673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. P., Kalaska J. F., Caminiti R., Massey J. T. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982 Nov;2(11):1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A. R., Houk J. C., Kohlerman N. J. Relation between red nucleus discharge and movement parameters in trained macaque monkeys. J Physiol. 1985 Jan;358:551–570. doi: 10.1113/jphysiol.1985.sp015566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. F., Thach W. T. Purkinje cell activity during motor learning. Brain Res. 1977 Jun 10;128(2):309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979 Dec;297(0):559–580. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. The natural discharges of Purkinje cells in paravermal regions of lobules V and VI of the monkey's cerebellum. J Physiol. 1977 Oct;271(2):515–536. doi: 10.1113/jphysiol.1977.sp012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano N., Yamamoto K. Simple-spike activity of cerebellar Purkinje cells related to visually guided wrist tracking movement in the monkey. J Neurophysiol. 1980 Mar;43(3):713–728. doi: 10.1152/jn.1980.43.3.713. [DOI] [PubMed] [Google Scholar]
- Miall R. C., Weir D. J., Stein J. F. Visuo-motor tracking during reversible inactivation of the cerebellum. Exp Brain Res. 1987;65(2):455–464. doi: 10.1007/BF00236319. [DOI] [PubMed] [Google Scholar]
- Miles F. A., Fuller J. H., Braitman D. J., Dow B. M. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980 May;43(5):1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Ritchie L. Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol. 1976 Nov;39(6):1246–1256. doi: 10.1152/jn.1976.39.6.1246. [DOI] [PubMed] [Google Scholar]
- Soechting J. F., Lacquaniti F. Invariant characteristics of a pointing movement in man. J Neurosci. 1981 Jul;1(7):710–720. doi: 10.1523/JNEUROSCI.01-07-00710.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks D. L. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 1986 Jan;66(1):118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970 Jul;33(4):527–536. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970 Jul;33(4):537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Timing of activity in cerebellar dentate nucleus and cerebral motor cortex during prompt volitional movement. Brain Res. 1975 May 2;88(2):233–241. doi: 10.1016/0006-8993(75)90387-x. [DOI] [PubMed] [Google Scholar]
- Uno M., Kozlovskaya I. B., Brooks V. B. Effects of cooling interposed nuclei on tracking-task performance in monkeys. J Neurophysiol. 1973 Nov;36(6):996–1003. doi: 10.1152/jn.1973.36.6.996. [DOI] [PubMed] [Google Scholar]


