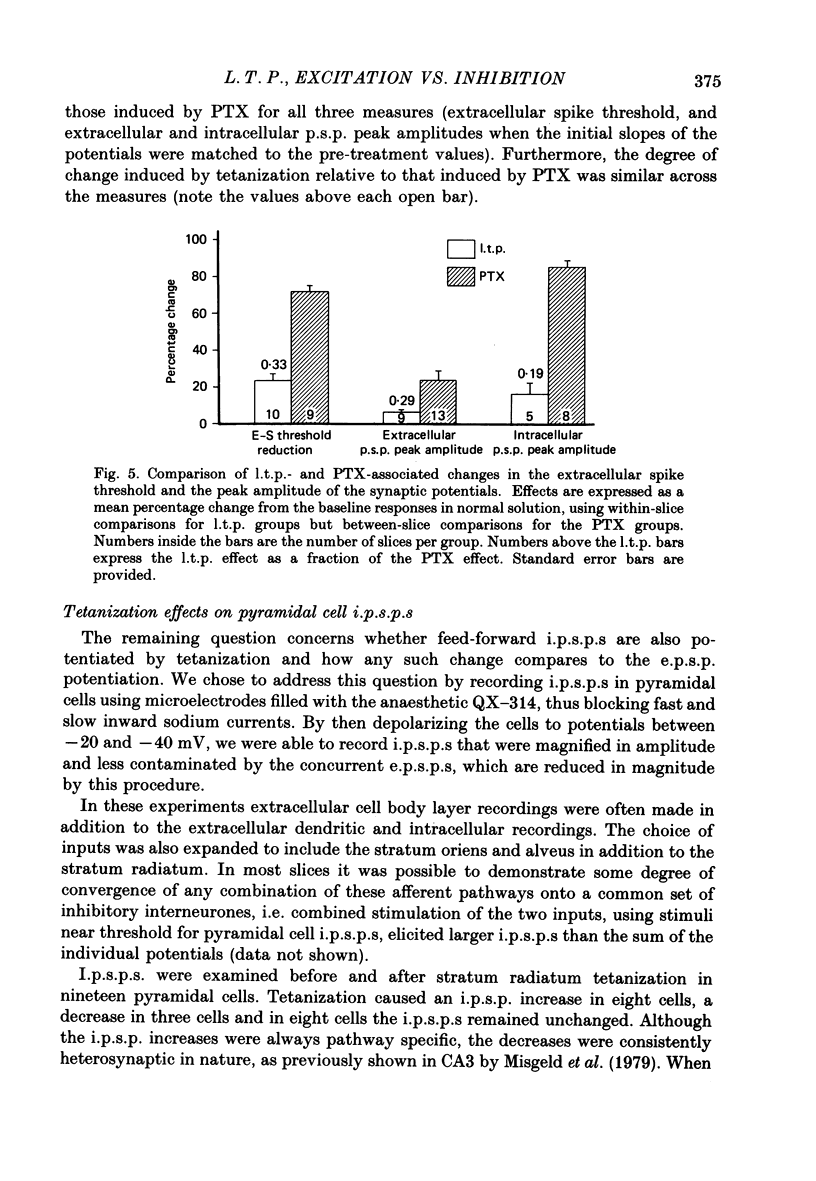
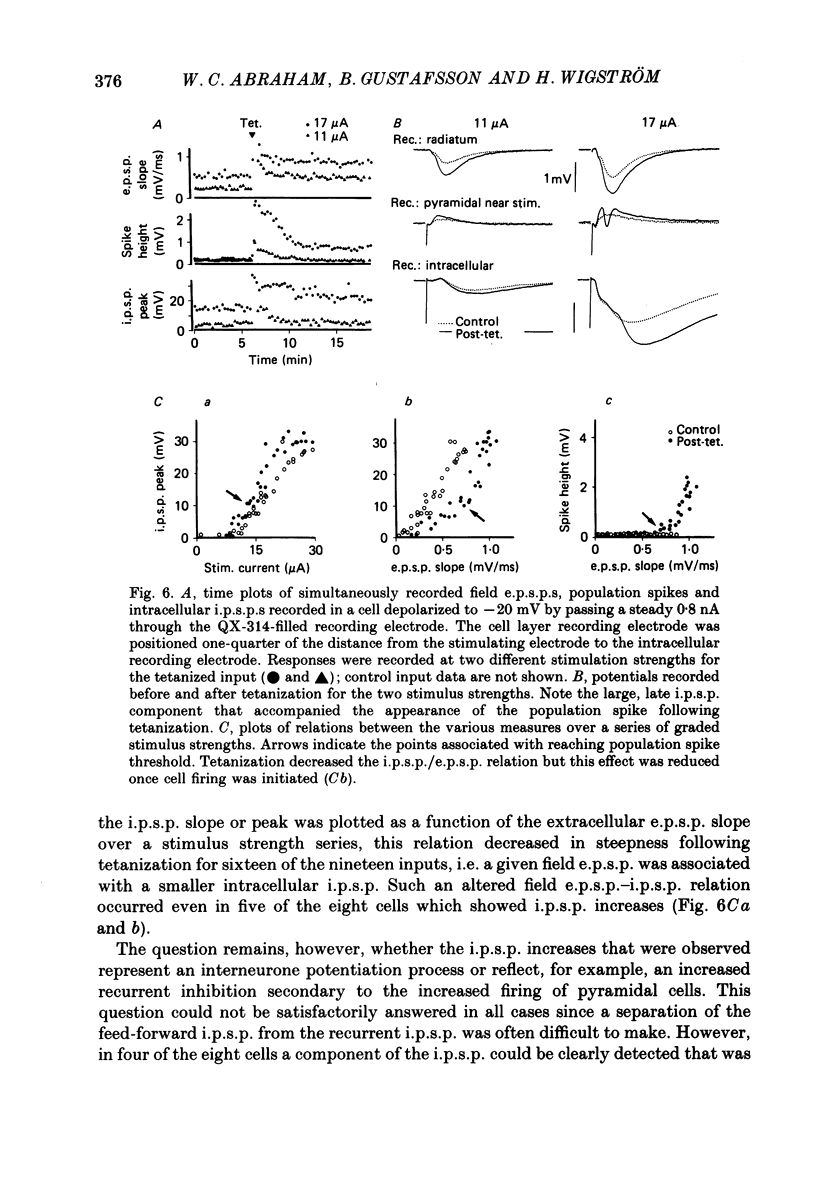
Abstract
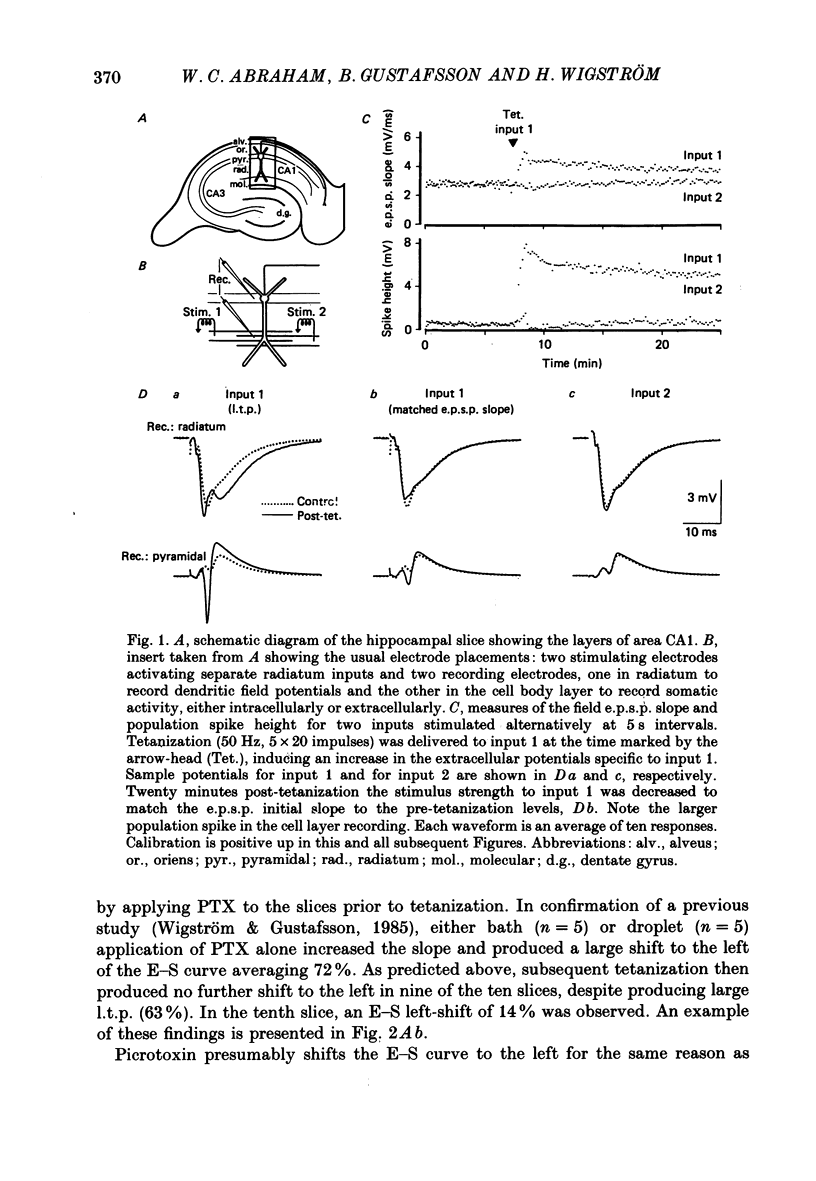
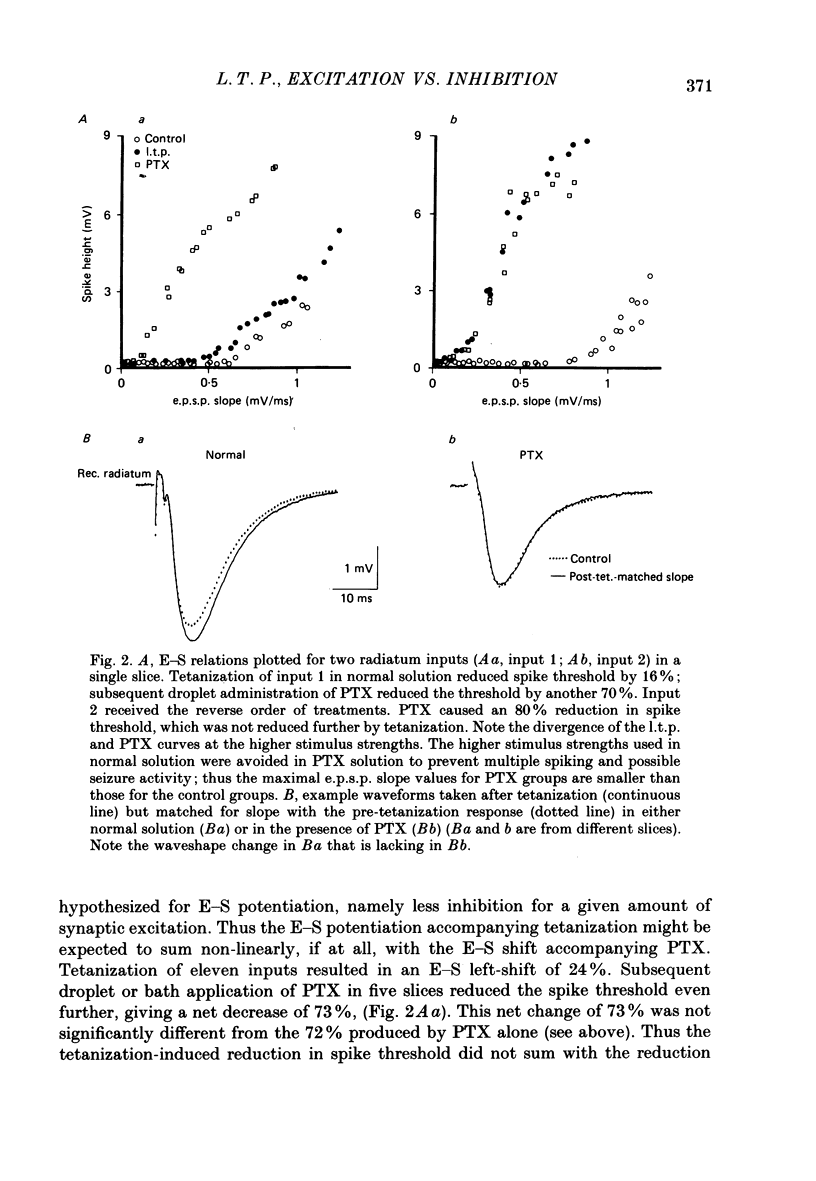
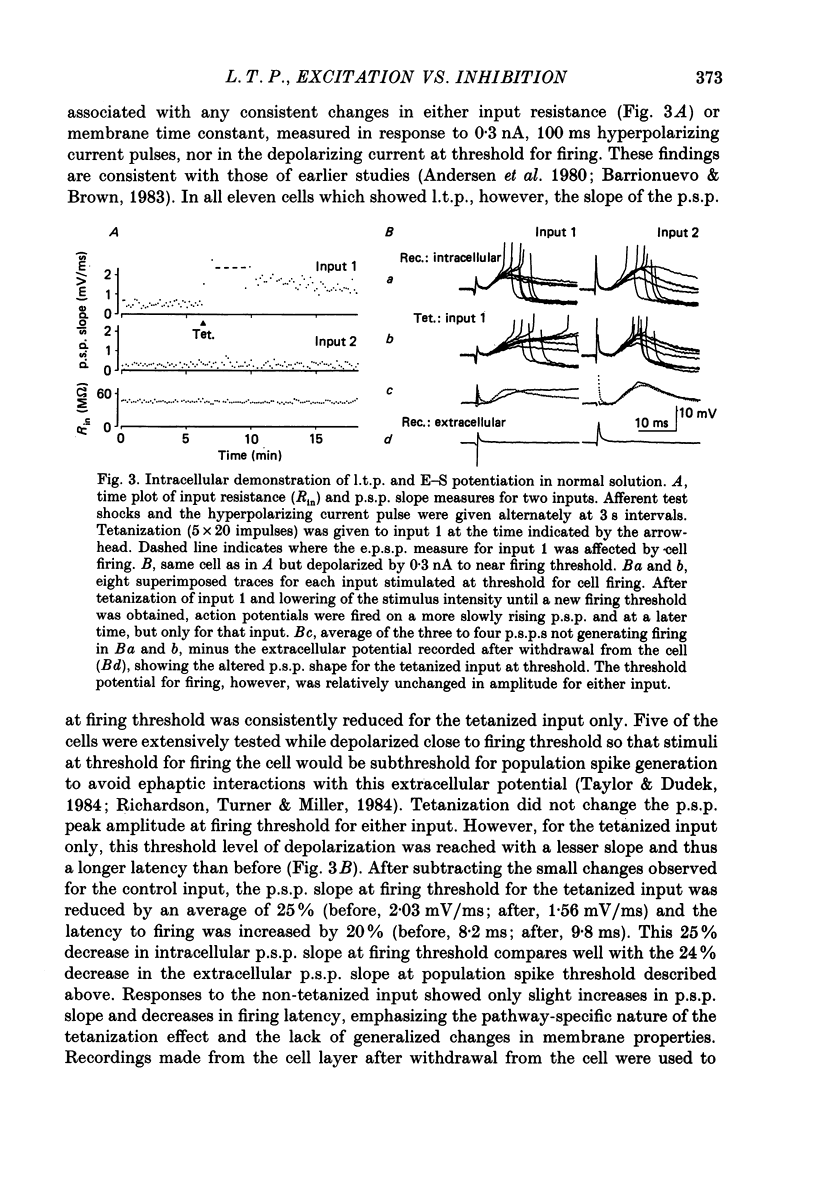
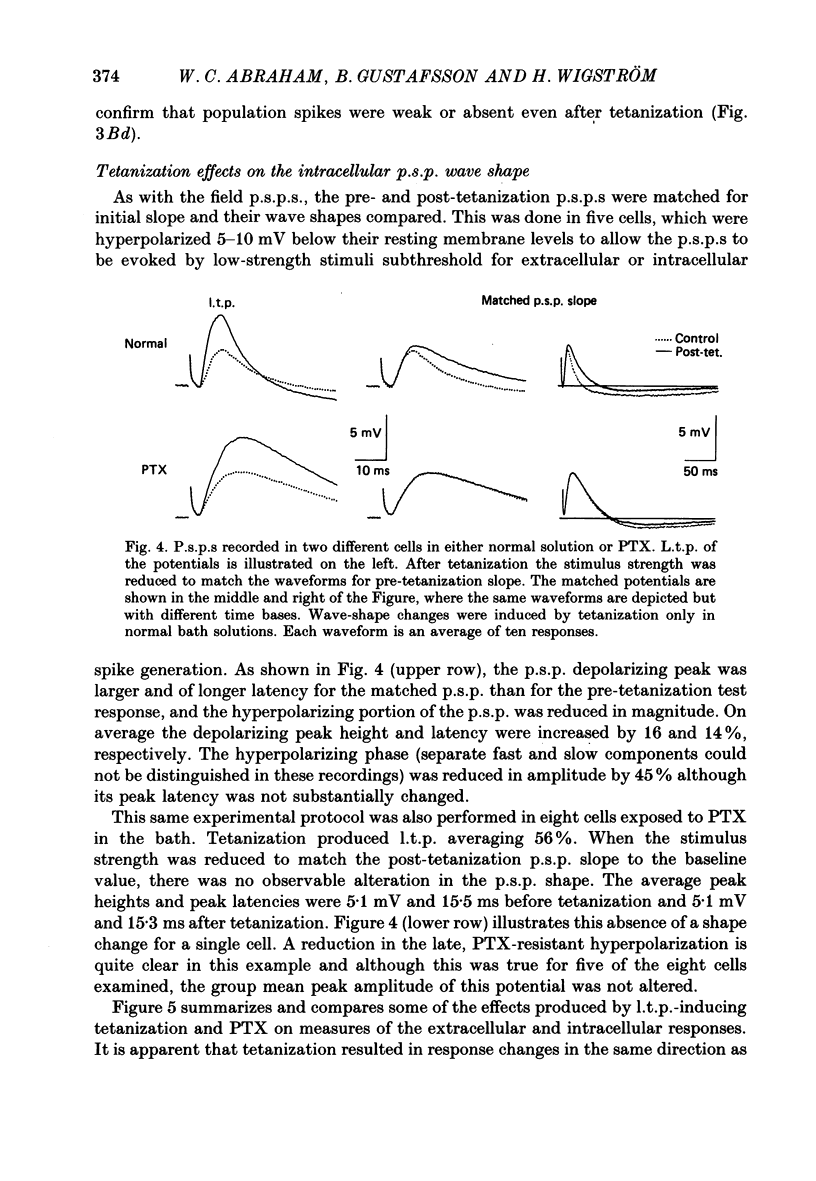
1. Tetanization of hippocampal pyramidal cell afferents travelling in stratum radiatum of area CA1 induces both long-term potentiation (l.t.p.) of extracellularly recorded excitatory postsynaptic potentials (e.p.s.p.s), and an increase in the number of cells firing, as measured by the extracellular population spike, for a given sized field e.p.s.p. The mechanism of this latter change, known as e.p.s.p.-spike (E-S) potentiation, was investigated in the guinea-pig hippocampal slice preparation. 2. Plots of the E-S relation before and after tetanization were constructed from measures taken over a series of stimulus strengths. Tetanization of afferents in stratum radiatum decreased the spike threshold by 24%, while the gamma-aminobutyric acid antagonist picrotoxin (PTX) decreased spike threshold by 72%. Sequential administration of PTX and tetanization, in either order, resulted in no more change in the E-S threshold than did PTX application alone. 3. Extracellular synaptic potentials, matched for initial slope before and after tetanization by adjusting the stimulus strength, showed an increased peak amplitude and increased peak latency following tetanization. PTX produced similar but larger percentage changes. Tetanization in the presence of PTX, however, did not alter the field potential wave shape. 4. Intracellular postsynaptic potentials (p.s.p.s) were also matched for initial slope before and after tetanization. Tetanization induced p.s.p. shape changes similar to those observed extracellularly, i.e. in the direction of less inhibition. Such changes did not occur in the presence of PTX. 5. Inhibitory p.s.p.s (i.p.s.p.s) were studied in depolarized pyramidal cells with microelectrodes filled with QX-314. Tetanization of afferents in stratum radiatum produced i.p.s.p. increases in eight of nineteen cells. These increases were generally attributable to an increased activity in the recurrent inhibitory pathway. Tetanization of the alveus failed to produce any lasting increases in the i.p.s.p. amplitude. 6. Tetanization of afferents in stratum radiatum decreased the ratio of the intracellular i.p.s.p. to field e.p.s.p. over stimulus strengths below population spike threshold. Above population spike threshold, the ratio tended towards its pretetanization level. 7. The results indicate that E-S potentiation results from an increase in the level of depolarization reached by a synaptic potential of given initial slope. These findings support the hypothesis that tetanization induces greater l.t.p. of excitatory inputs onto pyramidal cells than of inputs onto feed-forward inhibitory interneurones.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Bliss T. V., Goddard G. V. Heterosynaptic changes accompany long-term but not short-term potentiation of the perforant path in the anaesthetized rat. J Physiol. 1985 Jun;363:335–349. doi: 10.1113/jphysiol.1985.sp015714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Sundberg S. H., Sveen O., Swann J. W., Wigström H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol. 1980 May;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo G., Brown T. H. Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J Neurophysiol. 1982 Sep;48(3):597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Dingledine R., Gjerstad L. Penicillin blocks hippocampal IPSPs, unmasking prolonged EPSPs. Brain Res. 1979 May 18;168(1):205–209. doi: 10.1016/0006-8993(79)90141-0. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975 Mar 21;86(2):205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V., Riives M. Inhibitory modulation of long-term potentiation: evidence for a postsynaptic locus of control. Brain Res. 1982 May 27;240(2):259–272. doi: 10.1016/0006-8993(82)90221-9. [DOI] [PubMed] [Google Scholar]
- Griffith W. H., Brown T. H., Johnston D. Voltage-clamp analysis of synaptic inhibition during long-term potentiation in hippocampus. J Neurophysiol. 1986 Apr;55(4):767–775. doi: 10.1152/jn.1986.55.4.767. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Rose G. Long-term potentiation of excitatory synaptic transmission in the rat hippocampus: the role of inhibitory processes. J Physiol. 1982 Aug;329:541–552. doi: 10.1113/jphysiol.1982.sp014318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairiss E. W., Abraham W. C., Bilkey D. K., Goddard G. V. Field potential evidence for long-term potentiation of feed-forward inhibition in the rat dentate gyrus. Brain Res. 1987 Jan 13;401(1):87–94. doi: 10.1016/0006-8993(87)91167-x. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Sarvey J. M., Klee M. R. Heterosynaptic postactivation potentiation in hippocampal CA 3 neurons: long-term changes of the postsynaptic potentials. Exp Brain Res. 1979 Oct;37(2):217–229. doi: 10.1007/BF00237709. [DOI] [PubMed] [Google Scholar]
- Reymann K. G., Malisch R., Schulzeck K., Brödemann R., Ott T., Matthies H. The duration of long-term potentiation in the CA1 region of the hippocampal slice preparation. Brain Res Bull. 1985 Sep;15(3):249–255. doi: 10.1016/0361-9230(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Richardson T. L., Turner R. W., Miller J. J. Extracellular fields influence transmembrane potentials and synchronization of hippocampal neuronal activity. Brain Res. 1984 Mar 5;294(2):255–262. doi: 10.1016/0006-8993(84)91037-0. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. gamma-Aminobutyrate sensitivity does not change during long-term potentiation in rat hippocampal slices. Neuroscience. 1985 Jul;15(3):695–702. doi: 10.1016/0306-4522(85)90071-5. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Kunkel D. D. Morphology of identified interneurons in the CA1 regions of guinea pig hippocampus. J Comp Neurol. 1985 Feb 8;232(2):205–218. doi: 10.1002/cne.902320206. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Westgaard R. H. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Res. 1971 Dec 24;35(2):589–593. doi: 10.1016/0006-8993(71)90508-7. [DOI] [PubMed] [Google Scholar]
- Taylor C. P., Dudek F. E. Excitation of hippocampal pyramidal cells by an electrical field effect. J Neurophysiol. 1984 Jul;52(1):126–142. doi: 10.1152/jn.1984.52.1.126. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985 Sep;125(1):159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Wigström H., Swann J. W. Strontium supports synaptic transmission and long-lasting potentiation in the hippocampus. Brain Res. 1980 Jul 21;194(1):181–191. doi: 10.1016/0006-8993(80)91327-x. [DOI] [PubMed] [Google Scholar]
- Wilson R. C., Levy W. B., Steward O. Changes in translation of synaptic excitation to dentate granule cell discharge accompanying long-term potentiation. II. An evaluation of mechanisms utilizing dentate gyrus dually innervated by surviving ipsilateral and sprouted crossed temporodentate inputs. J Neurophysiol. 1981 Aug;46(2):339–355. doi: 10.1152/jn.1981.46.2.339. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Chujo T. Long-term potentiation in thin hippocampal sections studied by intracellular and extracellular recordings. Exp Neurol. 1978 Jan 15;58(2):242–250. doi: 10.1016/0014-4886(78)90137-1. [DOI] [PubMed] [Google Scholar]


