Abstract
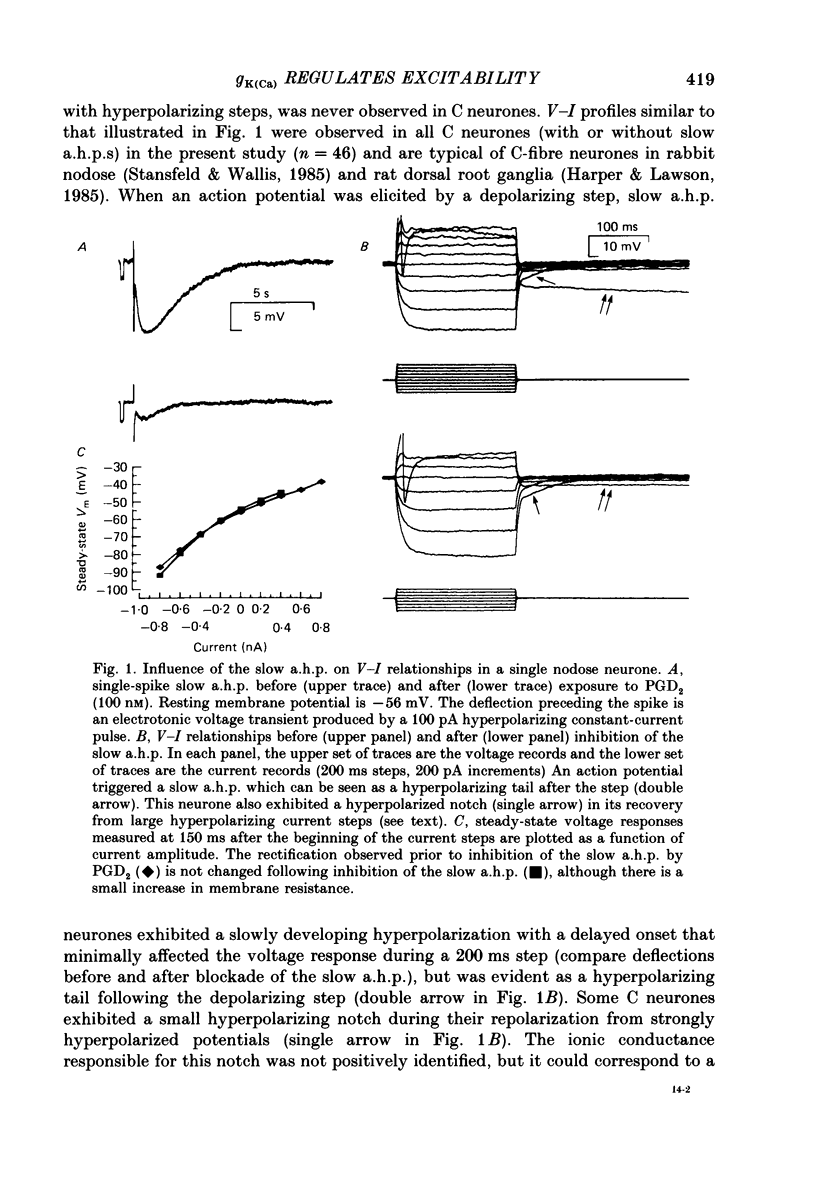
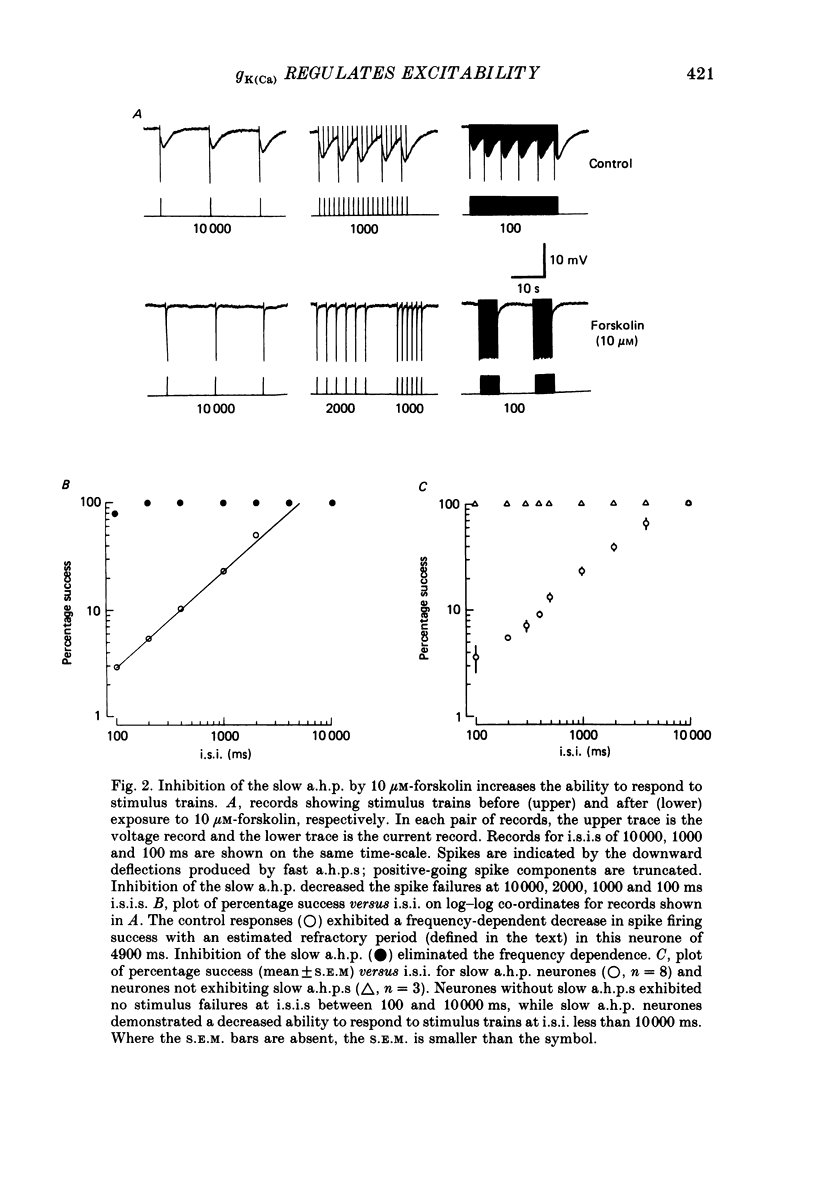
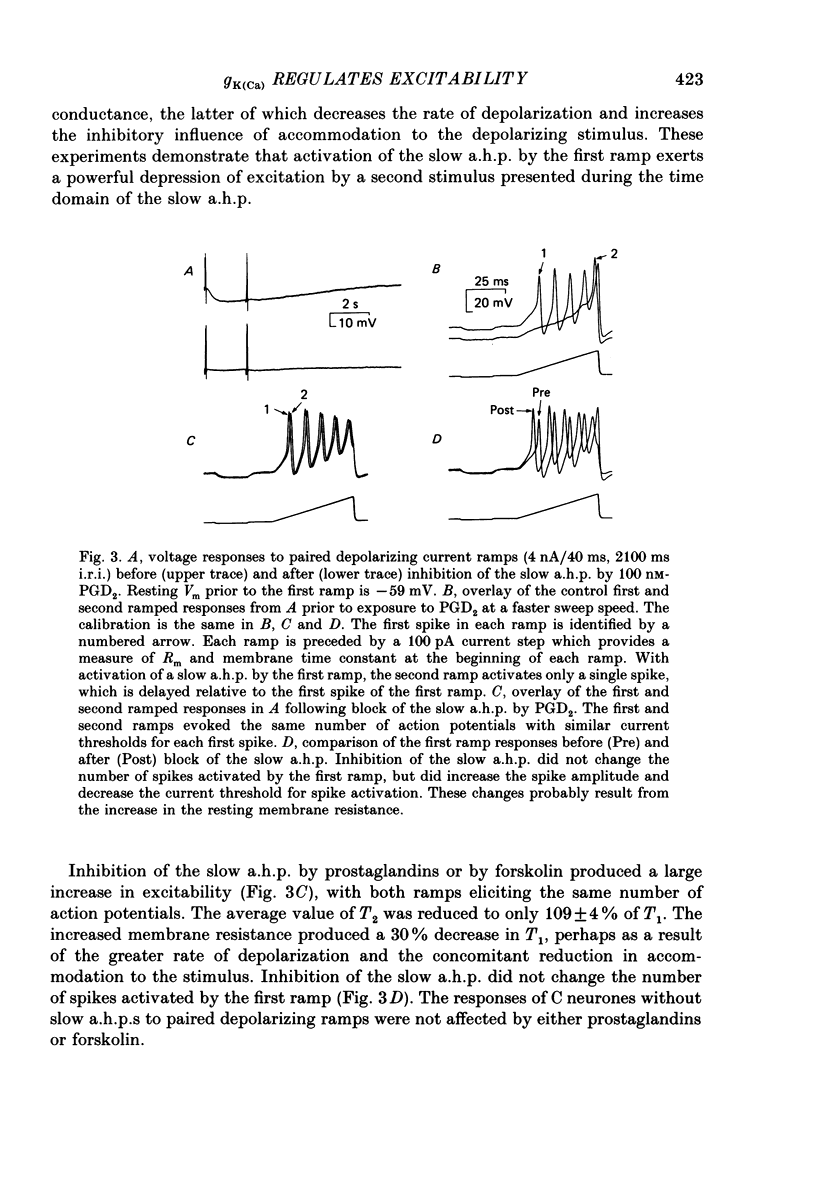
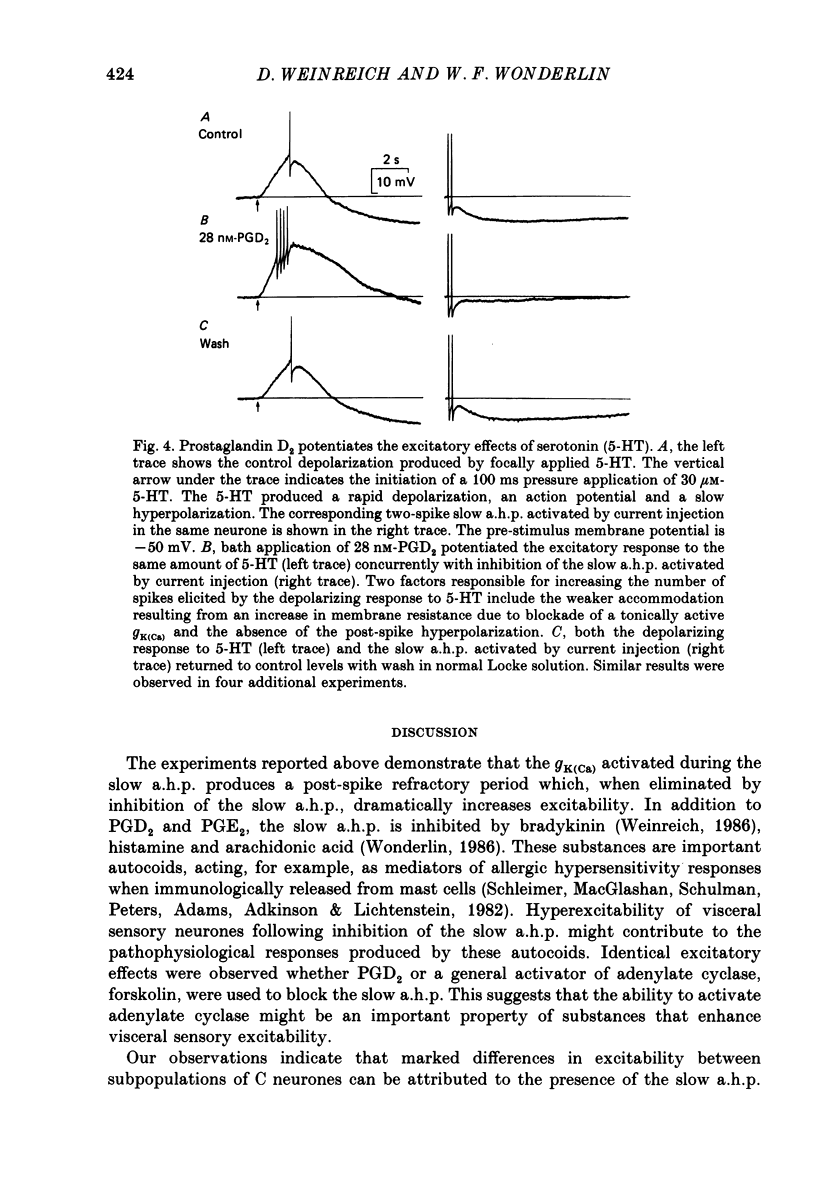
1. Conventional intracellular recordings were made from rabbit nodose neurones in vitro. Prostaglandins D2 and E2, but not F2 alpha, produced a selective, concentration-dependent (1-100 nM) inhibition of a slow, Ca2+-dependent spike after-hyperpolarization (a.h.p.). Block of the slow a.h.p. was accompanied by an increased membrane resistance and a small (less than 10 mV) depolarization of the membrane potential. Inhibition of the slow a.h.p. produced no change in the voltage-current relationship other than the increased membrane resistance. 2. In C neurones with slow a.h.p.s, trains of brief depolarizing current pulses (2 ms duration, 0.1-10 Hz) could not elicit repetitive action potentials without failure at rates above 0.1 Hz. By contrast, C neurones without slow a.h.p.s could respond at stimulus frequencies up to 10 Hz. The frequency-dependent spike firing ability of slow a.h.p. neurones was eliminated by inhibition of the slow a.h.p. 3. Action potentials were also evoked by intrasomatic injection of paired, depolarizing current ramps (1 nA/10 ms, 0.1-5 s inter-ramp interval). For neurones without a slow a.h.p., the current threshold and number of evoked spikes were the same for both ramps, and the ramps were nearly superimposable. In neurones with a slow a.h.p., the current threshold for the first spike in the second ramp was greatly increased (300-500%) and the number of evoked spikes was reduced. Following inhibition of the slow a.h.p., the current threshold and number of evoked spikes was the same for both ramps. 4. Forskolin, a direct activator of the catalytic subunit of adenylate cyclase, also produced a concentration-dependent inhibition of the slow a.h.p., with 50% block at 30 nM. Prostaglandin D2 and forskolin produced identical enhancement of excitability in C neurones and neither substance produced any effect on C neurones that could not be attributed to inhibition of the Ca2+-dependent K+ conductance associated with the slow a.h.p. We propose that, in some visceral sensory neurones, the level of excitability is regulated by cyclic AMP-mediated control of the slow a.h.p.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccaglini P. I., Hogan P. G. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):594–598. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F., Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol Scand. 1974 Sep;92(1):27–47. doi: 10.1111/j.1748-1716.1974.tb05720.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F., Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course. First interval firing. Acta Physiol Scand. 1974 Aug;91(4):528–544. doi: 10.1111/j.1748-1716.1974.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C., Gallego R. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. J Physiol. 1983 Sep;342:603–614. doi: 10.1113/jphysiol.1983.sp014871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C., Baker D. G., Ginzel K. H., Morrison M. A. Comparison of the effects of histamine and prostaglandin on afferent C-fiber endings and irritant receptors in the intrapulmonary airways. Adv Exp Med Biol. 1978;99:291–305. doi: 10.1007/978-1-4613-4009-6_32. [DOI] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C., Ginzel K. H., Baker D. G., Banzett R. B., Morrison M. A. Stimulation of 'irritant' receptors and afferent C-fibres in the lungs by prostaglandins. Nature. 1976 Dec 2;264(5585):451–453. doi: 10.1038/264451a0. [DOI] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Single-channel analysis of fast transient potassium currents from rat nodose neurones. J Physiol. 1985 Dec;369:199–208. doi: 10.1113/jphysiol.1985.sp015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J. Inhibition of ACH release by prostaglandin E1 in the rabbit superior cervical ganglion. Neuropharmacology. 1980 Nov;19(11):1137–1140. doi: 10.1016/0028-3908(80)90114-8. [DOI] [PubMed] [Google Scholar]
- Fowler J. C., Greene R., Weinreich D. Two calcium-sensitive spike after-hyperpolarizations in visceral sensory neurones of the rabbit. J Physiol. 1985 Aug;365:59–75. doi: 10.1113/jphysiol.1985.sp015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. C., Wonderlin W. F., Weinreich D. Prostaglandins block a Ca2+-dependent slow spike afterhyperpolarization independent of effects on Ca2+ influx in visceral afferent neurons. Brain Res. 1985 Oct 21;345(2):345–349. doi: 10.1016/0006-8993(85)91014-5. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985 Feb;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Morita K., North R. A. Calcium-dependent after-potentials in visceral afferent neurones of the rabbit. J Physiol. 1984 Oct;355:479–492. doi: 10.1113/jphysiol.1984.sp015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe R. A., Sampson S. R. Analysis of passive and active electrophysiologic properties of neurons in mammalian nodose ganglia maintained in vitro. J Neurophysiol. 1976 Jul;39(4):802–815. doi: 10.1152/jn.1976.39.4.802. [DOI] [PubMed] [Google Scholar]
- Kimura H., Okamoto K., Sakai Y. Modulatory effects of prostaglandin D2, E2 and F2 alpha on the postsynaptic actions of inhibitory and excitatory amino acids in cerebellar Purkinje cell dendrites in vitro. Brain Res. 1985 Mar 25;330(2):235–244. doi: 10.1016/0006-8993(85)90682-1. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Van Ranst L., Verhofstad A. A. Ultrastructural localization of serotonin in the intrapulmonary neuroepithelial bodies of neonatal rabbits by use of immunoelectron microscopy. Cell Tissue Res. 1986;243(3):455–459. doi: 10.1007/BF00218051. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Nemeth P. R., Zafirov D., Wood J. D. Forskolin mimics slow synaptic excitation in myenteric neurons. Eur J Pharmacol. 1984 Jun 1;101(3-4):303–304. doi: 10.1016/0014-2999(84)90176-6. [DOI] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Shimizu T., Mizuno N., Amano T., Hayaishi O. Prostaglandin D2, a neuromodulator. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6231–6234. doi: 10.1073/pnas.76.12.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld C. E., Wallis D. I. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. J Neurophysiol. 1985 Aug;54(2):245–260. doi: 10.1152/jn.1985.54.2.245. [DOI] [PubMed] [Google Scholar]
- Weinreich D. Bradykinin inhibits a slow spike afterhyperpolarization in visceral sensory neurons. Eur J Pharmacol. 1986 Dec 2;132(1):61–63. doi: 10.1016/0014-2999(86)90010-5. [DOI] [PubMed] [Google Scholar]


