Abstract
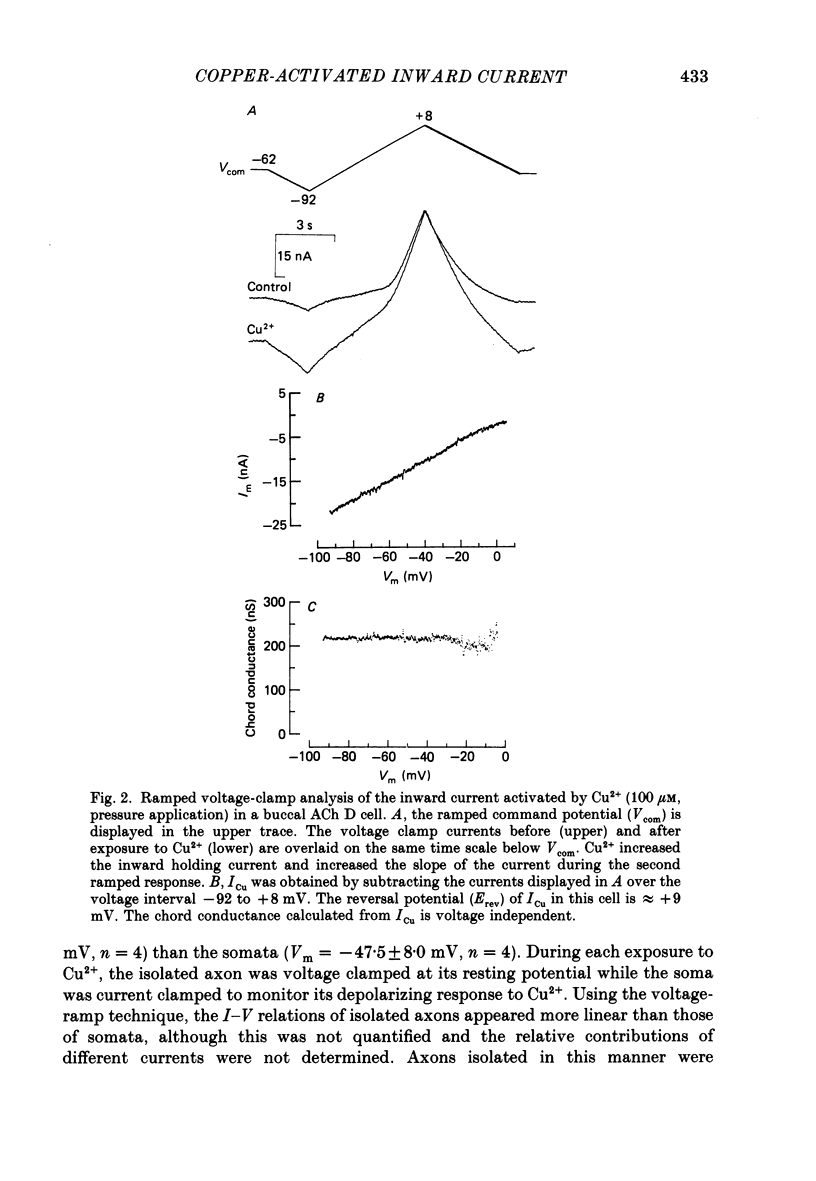
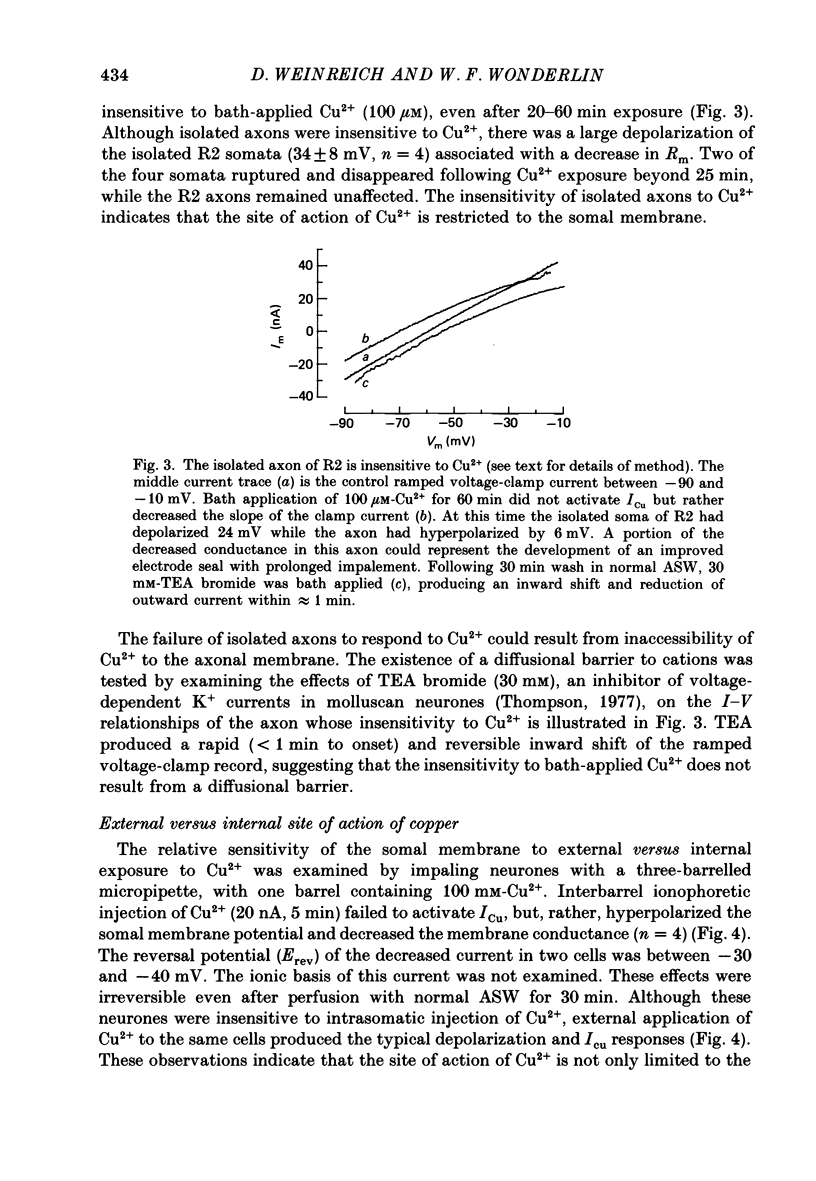
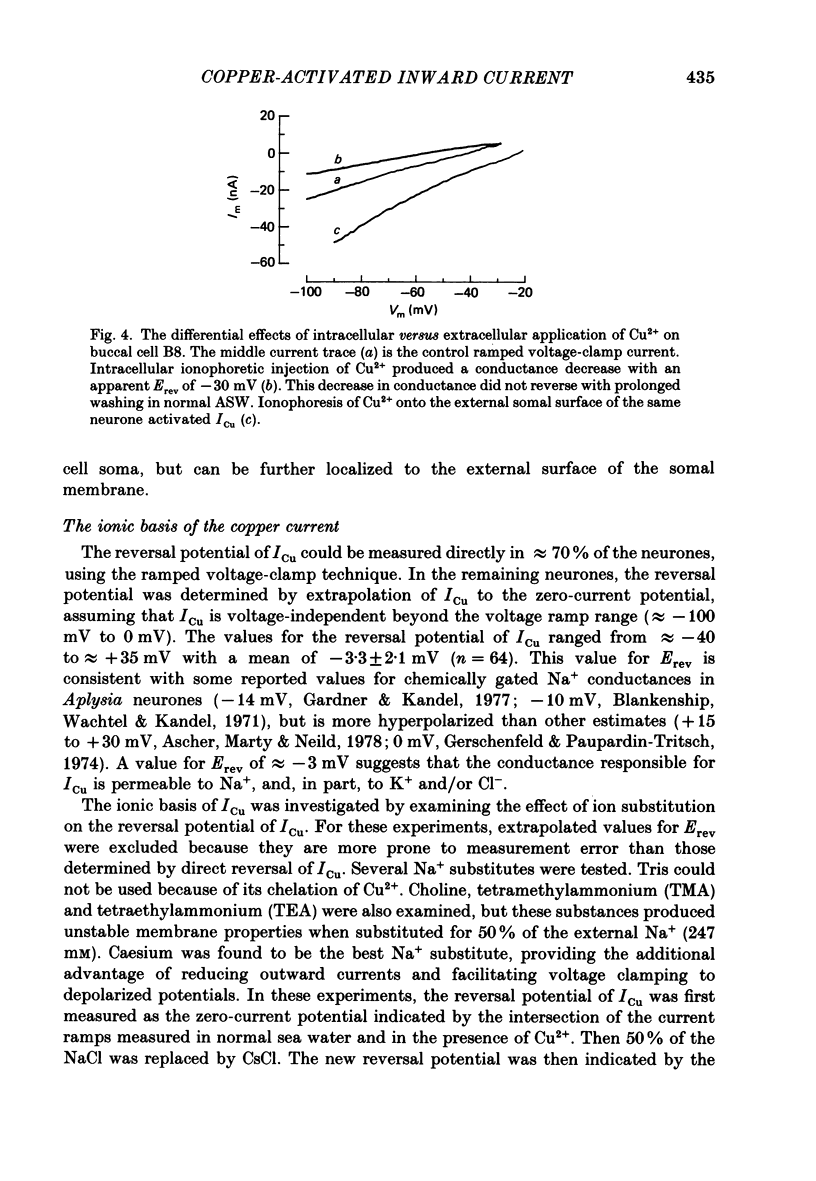
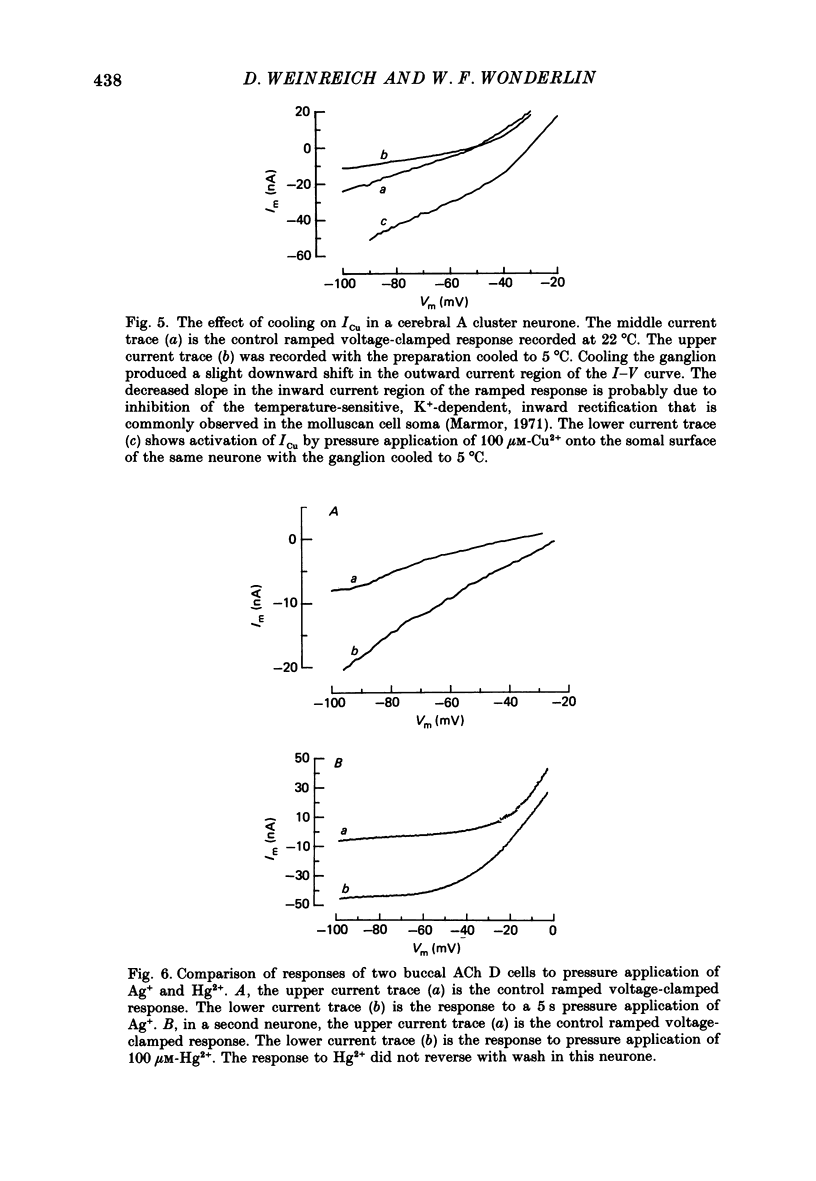
1. Reidentifiable Aplysia neurones were current and voltage clamped in vitro using standard microelectrode techniques. 2. Bath or focal application of Cu2+ at concentrations of 1-100 microM produced a rapid and reversible depolarization of the somal, but not the axonal, membrane potential. The depolarization was accompanied by an increased membrane conductance and activation of an inward current (ICu) which could not be activated by intracellular ionophoretic injection of Cu2+. 3. ICu is carried, in part, by Na+ because the reversal potential of ICu was shifted in a Nernstian fashion by decreasing the extracellular Na+ concentration. The reversal potential of ICu was not affected by removal of extracellular Ca2+ or K+. 4. ICu does not result from (1) activation of known chemically or voltage-gated Na+ conductances, (2) inhibition of the Na+-K+-ATPase or (3) a generalized increase in membrane permeability resulting from lipid peroxidation. 5. A similar inward current was activated by AgNO3 (100 microM) and HgCl2 (100 microM).
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Arhem P. Effects of some heavy metal ions on the ionic currents of myelinated fibres from Xenopus laevis. J Physiol. 1980 Sep;306:219–231. doi: 10.1113/jphysiol.1980.sp013393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. E., Wachtel H., Kandel E. R. Ionic mechanisms of excitatory, inhibitory, and dual synaptic actions mediated by an identified interneuron in abdominal ganglion of Aplysia. J Neurophysiol. 1971 Jan;34(1):76–92. doi: 10.1152/jn.1971.34.1.76. [DOI] [PubMed] [Google Scholar]
- Bowler K., Duncan C. J. The effect of copper on membrane enzymes. Biochim Biophys Acta. 1970 Jan 6;196(1):116–119. doi: 10.1016/0005-2736(70)90174-4. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Swann J. W., Yarowsky P. J. Effect of curare on responses to different putative neurotransmitters in Aplysia neurons. J Neurobiol. 1977 Mar;8(2):119–132. doi: 10.1002/neu.480080204. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Kerkut G. A., Walker R. J. Ramped voltage clamp study of the action of acetylcholine on three types of neurons in the snail (Helix aspersa) brain. Comp Biochem Physiol C. 1979;63C(2):269–278. doi: 10.1016/0306-4492(79)90073-x. [DOI] [PubMed] [Google Scholar]
- Colmers W. F., Lewis D. V., Jr, Wilson W. A. Cs+ loading reveals Na+-dependent persistent inward current and negative slope resistance region in Aplysia giant neurons. J Neurophysiol. 1982 Nov;48(5):1191–1200. doi: 10.1152/jn.1982.48.5.1191. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Effects of copper on cortical neurones. Brain Res. 1969 May;13(3):607–611. doi: 10.1016/0006-8993(69)90272-8. [DOI] [PubMed] [Google Scholar]
- Epstein P. S., McIlwain H. Actions of cupric salts on isolated cerebral tissues. Proc R Soc Lond B Biol Sci. 1966 Dec 13;166(1004):295–302. doi: 10.1098/rspb.1966.0100. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Klee M. R. Strychnine interactions with acetylcholine, dopamine and serotonin receptors in Aplysia neurons. Brain Res. 1974 Jan 4;65(1):109–126. doi: 10.1016/0006-8993(74)90339-4. [DOI] [PubMed] [Google Scholar]
- GURD F. R., WILCOX P. E. Complex formation between metallic cations and proteins, peptides and amino acids. Adv Protein Chem. 1956;11:311–427. doi: 10.1016/s0065-3233(08)60424-6. [DOI] [PubMed] [Google Scholar]
- Gardner D., Kandel E. R. Physiological and kinetic properties of cholinergic receptors activated by multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol. 1977 Mar;40(2):333–348. doi: 10.1152/jn.1977.40.2.333. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Steady-state contribution of the sodium pump to the resting potential of a molluscan neurone. J Physiol. 1974 Oct;242(1):35–48. doi: 10.1113/jphysiol.1974.sp010692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R., Connor J. A. A study of the effects of p-chloromercuribenzene sulfonic acid on acetylcholine-induced responses of molluscan neurons. J Pharmacol Exp Ther. 1976 Jul;198(1):146–154. [PubMed] [Google Scholar]
- Hanlon D. P., Watt D. S., Westhead E. W. The interaction of divalent metal ions with tris buffer in dilute solution. Anal Biochem. 1966 Aug;16(2):225–233. doi: 10.1016/0003-2697(66)90150-3. [DOI] [PubMed] [Google Scholar]
- Hexum T. D. Studies on the reaction catalyzed by transport (Na, K) adenosine triphosphatase. I. Effects of divalent metals. Biochem Pharmacol. 1974 Dec 15;23(24):3441–3447. doi: 10.1016/0006-2952(74)90347-5. [DOI] [PubMed] [Google Scholar]
- Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966 Jun 18;210(5042):1220–1222. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- Kado R. T. Aplysia giant cell: soma-axon voltage clamp current differences. Science. 1973 Nov 23;182(4114):843–845. doi: 10.1126/science.182.4114.843. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K. S., Rowse C., Hochstein P. Copper-induced generation of superoxide in human red cell membrane. Biochem Biophys Res Commun. 1978 Jul 28;83(2):587–592. doi: 10.1016/0006-291x(78)91030-6. [DOI] [PubMed] [Google Scholar]
- Marmor M. F. The effects of temperature and ions on the current-voltage relation and electrical characteristics of a molluscan neurone. J Physiol. 1971 Nov;218(3):573–598. doi: 10.1113/jphysiol.1971.sp009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman R. E., Weinreich D. On the nature of histamine-mediated slow hyperpolarizing synaptic potentials in identified molluscan neurones. J Physiol. 1982 Jul;328:485–506. doi: 10.1113/jphysiol.1982.sp014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. M. Effects of sulfhydryl blockade on axonal function. J Cell Physiol. 1958 Apr;51(2):161–171. doi: 10.1002/jcp.1030510203. [DOI] [PubMed] [Google Scholar]
- Schroeder H. A., Nason A. P., Tipton I. H., Balassa J. J. Essential trace metals in man: copper. J Chronic Dis. 1966 Sep;19(9):1007–1034. doi: 10.1016/0021-9681(66)90033-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E. Effect of procaine on electrical properties of squid axon membrane. Am J Physiol. 1959 May;196(5):1071–1078. doi: 10.1152/ajplegacy.1959.196.5.1071. [DOI] [PubMed] [Google Scholar]
- Tauc L. Transmission in invertebrate and vertebrate ganglia. Physiol Rev. 1967 Jul;47(3):521–593. doi: 10.1152/physrev.1967.47.3.521. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting-Beall H. P., Clark D. A., Suelter C. H., Wells W. W. Studies on the interaction of chick brain microsomal (Na+ + K+)-ATPase with copper. Biochim Biophys Acta. 1973 Jan 2;291(1):229–236. doi: 10.1016/0005-2736(73)90414-8. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]
- del CASTILLO-NICOLAU J., HUFSCHMIDT H. J. Reversible poisoning of nerve fibers by heavy-metal ions. Nature. 1951 Jan 27;167(4239):146–147. doi: 10.1038/167146b0. [DOI] [PubMed] [Google Scholar]


