Abstract
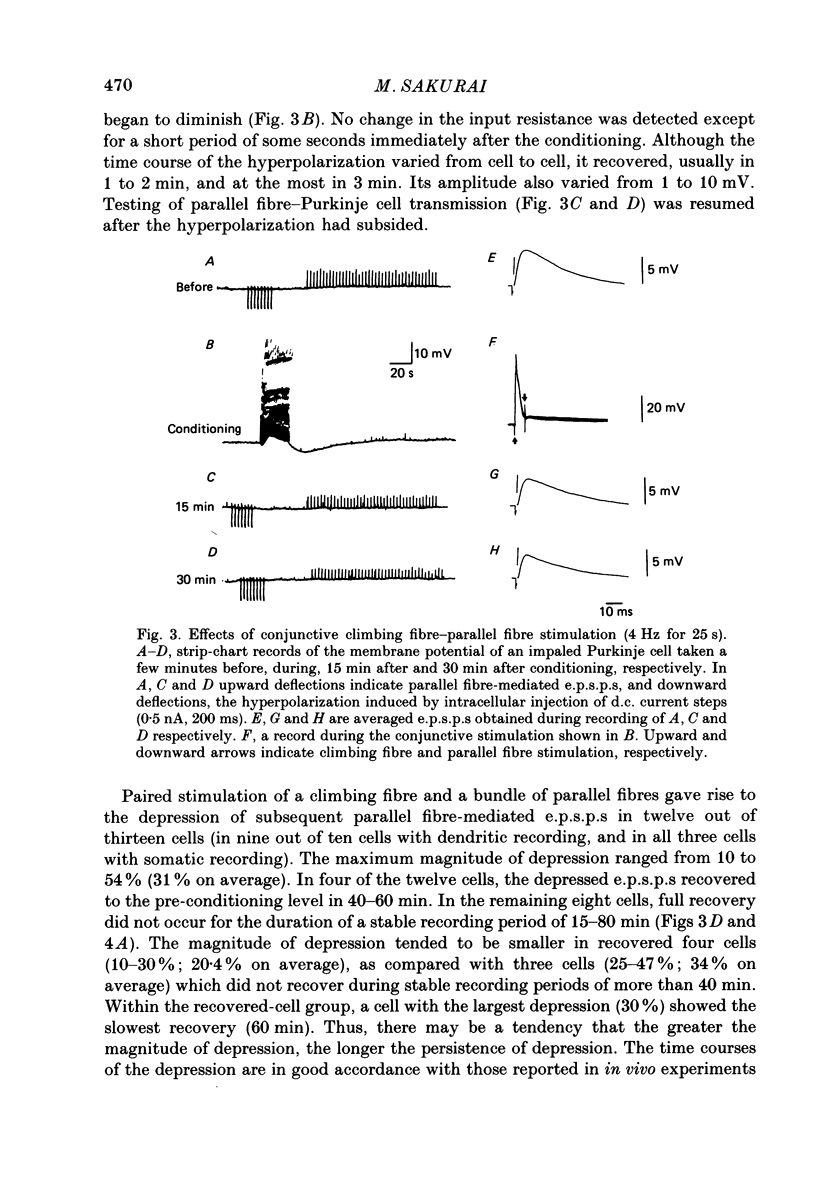
1. Synaptic transmission from parallel fibres to Purkinje cells and its modification by paired stimulation of parallel fibres and climbing fibres were studied in in vitro slices of the cerebellum obtained from guinea-pigs. 2. Intracellular recordings were made from Purkinje cells, mainly from dendrites in the middle third of the molecular layer, but also, in a few cases, from somata. Climbing fibres were activated by stimulation of the white matter, while parallel fibres were stimulated with an electrode placed near the pial surface of the molecular layer. 3. Stimulation of the white matter elicited antidromic spikes, all-or-none climbing fibre responses, disynaptic responses through mossy fibres and parallel fibres, and trisynaptic responses through inhibitory interneurones. Climbing fibre responses were often followed by a small plateau potential, usually less than 2-3 mV in amplitude and less than 100 ms in duration, followed by a slow hyperpolarization which reached its peak in several seconds. Inhibitory inputs to Purkinje cells were blocked with picrotoxin for the experiments described below. 4. Stimulation of the superficial molecular layer with currents less than 50 microA produced graded parallel fibre-mediated excitatory postsynaptic potentials (e.p.s.p.s) ranging from 4 to 8 mV in peak amplitude. 5. Conjunctive stimulation of climbing fibres and parallel fibres at 4 Hz for 25 s induced depression of parallel fibre-mediated e.p.s.p.s in Purkinje cells, both in the peak amplitudes and in the slopes. The depression was about 30% on average and lasted for more than 50 min. 6. No such depression occurred when the intensity of the white matter stimulation was set just subthreshold for the climbing fibre innervating the Purkinje cell under study. Instead, the parallel fibre-mediated e.p.s.p.s were moderately potentiated for a period ranging from 10 to 50 min. Repetitive stimulation of the climbing fibre alone did not affect parallel fibre-mediated e.p.s.p.s. 7. Immediately after the conjunctive stimulation or the repetitive stimulation of climbing fibres alone, a transient hyperpolarization which lasted for several minutes was seen. Its time course was similar to that of the hyperpolarization following a climbing fibre response. Except for this, there were no associated changes in the membrane potential, the input resistance, or the magnitudes of climbing fibre responses in any of the cases mentioned in 5 and 6 above.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Campbell N. C., Ekerot C. F., Hesslow G. Interaction between responses in Purkinje cells evoked by climbing fibre impulses and parallel fibre volleys in the cat. J Physiol. 1983 Jul;340:225–238. doi: 10.1113/jphysiol.1983.sp014760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N. C., Ekerot C. F., Hesslow G., Oscarsson O. Dendritic plateau potentials evoked in Purkinje cells by parallel fibre volleys in the cat. J Physiol. 1983 Jul;340:209–223. doi: 10.1113/jphysiol.1983.sp014759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N. C., Hesslow G. Plateau potentials evoked by climbing-fibre stimulation are restricted to the Purkinje cell dendrites of the cat. Neurosci Lett. 1984 Mar 23;45(2):187–192. doi: 10.1016/0304-3940(84)90097-1. [DOI] [PubMed] [Google Scholar]
- Crepel F., Delhaye-Bouchaud N. Intracellular analyses of synaptic potentials in cerebellar Purkinje cells of the rat. Brain Res. 1978 Oct 20;155(1):176–181. doi: 10.1016/0006-8993(78)90321-9. [DOI] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Garthwaite J. Morphological and electrophysiological characteristics of rat cerebellar slices maintained in vitro. J Physiol. 1981 Jul;316:127–138. doi: 10.1113/jphysiol.1981.sp013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The inhibitory interneurones within the cerebellar cortex. Exp Brain Res. 1966;1(1):1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966;1(1):82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985 Sep 9;342(2):357–360. doi: 10.1016/0006-8993(85)91136-9. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Oscarsson O. Prolonged depolarization elicited in Purkinje cell dendrites by climbing fibre impulses in the cat. J Physiol. 1981 Sep;318:207–221. doi: 10.1113/jphysiol.1981.sp013859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Adaptive filter model of the cerebellum. Biol Cybern. 1982;45(3):195–206. doi: 10.1007/BF00336192. [DOI] [PubMed] [Google Scholar]
- Ghelarducci B., Ito M., Yagi N. Impulse discharges from flocculus Purkinje cells of alert rabbits during visual stimulation combined with horizontal head rotation. Brain Res. 1975 Apr 4;87(1):66–72. doi: 10.1016/0006-8993(75)90780-5. [DOI] [PubMed] [Google Scholar]
- Gilbert P. F., Thach W. T. Purkinje cell activity during motor learning. Brain Res. 1977 Jun 10;128(2):309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972 May 12;40(1):81–84. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- Ito M., Sakurai M., Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982 Mar;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Okamoto K., Sakai Y. Climbing and parallel fiber responses recorded intracellularly from Purkinje cell dendrites in guinea pig cerebellar slices. Brain Res. 1985 Dec 2;348(2):213–219. doi: 10.1016/0006-8993(85)90439-1. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Walton K., Hillman D. E., Sotelo C. Inferior olive: its role in motor learing. Science. 1975 Dec 19;190(4220):1230–1231. doi: 10.1126/science.128123. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y., Sugimori M. Isolated mammalian brain in vitro: new technique for analysis of electrical activity of neuronal circuit function. Fed Proc. 1981 Jun;40(8):2240–2245. [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles F. A., Lisberger S. G. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- Rawson J. A., Tilokskulchai K. Climbing fibre modification of cerebellar Purkinje cell responses to parallel fibre inputs. Brain Res. 1982 Apr 15;237(2):492–497. doi: 10.1016/0006-8993(82)90461-9. [DOI] [PubMed] [Google Scholar]
- Thompson R. F. The neurobiology of learning and memory. Science. 1986 Aug 29;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Yamamoto C. Electrical activity observed in vitro in thin sections from guinea-pig cerebellum. Jpn J Physiol. 1974 Apr;24(2):177–188. doi: 10.2170/jjphysiol.24.177. [DOI] [PubMed] [Google Scholar]
- Yeo C. H., Hardiman M. J., Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res. 1985;60(1):99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]




