Abstract
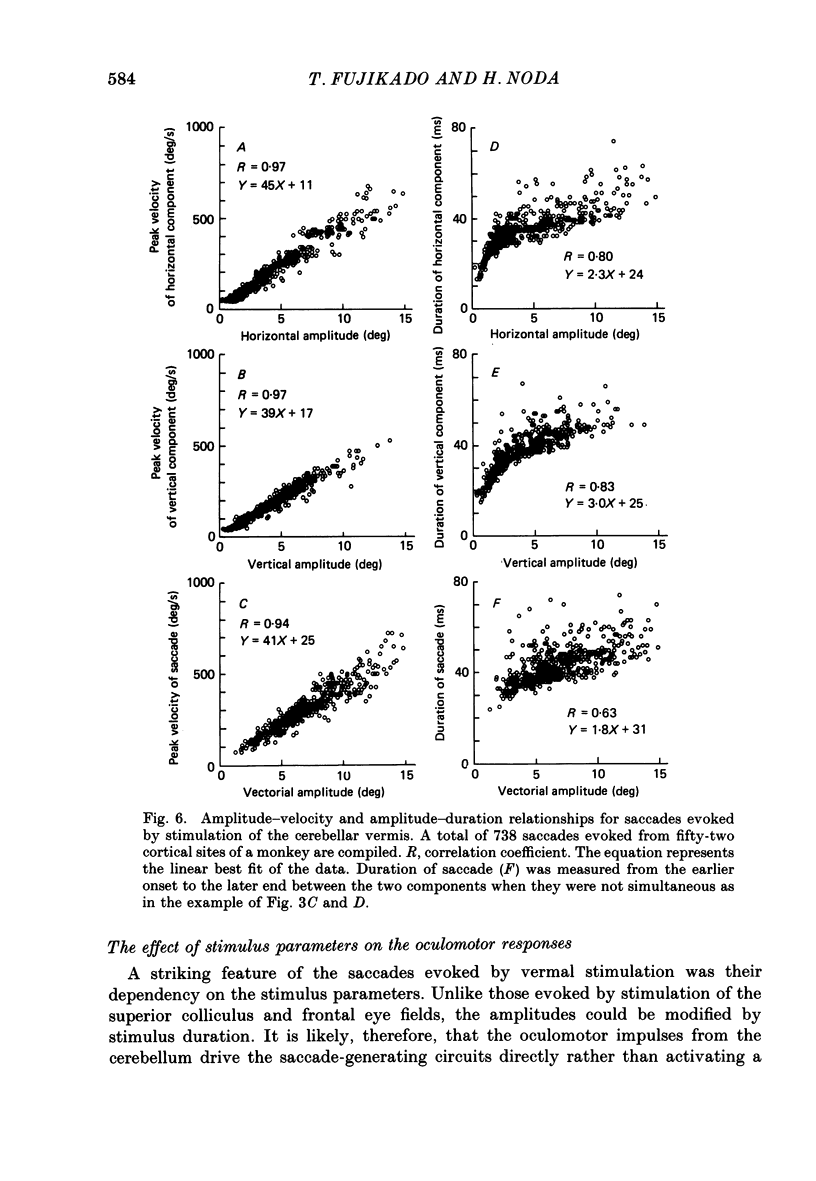
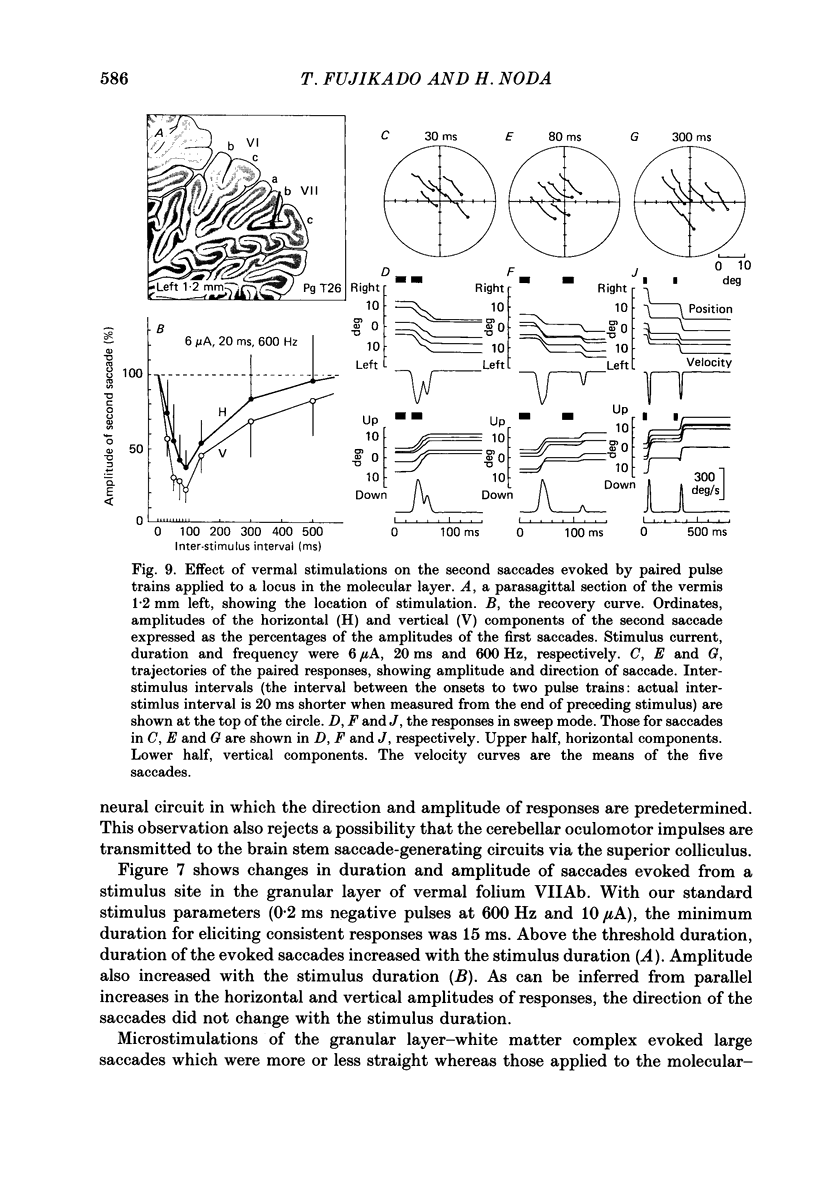
1. Oculomotor responses to microstimulation of the cerebellar vermis were studied on macaque monkeys by measuring eye position with a magnetic search-coil method. 2. Vermal microstimulation resulted in conjugate eye movements. Analyses of amplitude-velocity and amplitude-duration relationships revealed that although the peak velocities were slightly faster, the durations of these responses were comparable to those of spontaneous and visually guided saccadic eye movements (saccades). 3. Systematic mapping with microstimulation revealed that the low-threshold sites were localized in the white matter of a limited number of folia in the posterior vermis. The low-threshold region from which saccades could be evoked with stimulus intensities less than 10 microA was confined to lobule VII in seven monkeys; in the other five monkeys it included a posterior part of lobule VI (folium VIc). This region coincided with the distribution of saccade-related neural activity observed in the present study and corresponded to the vermal folia from which we previously recorded the burst mossy fibre units and the oculomotor Purkinje cell activity (Kase, Miller & Noda, 1980). 4. Microstimulation of most sites in the oculomotor vermis evoked saccades in oblique directions and the horizontal component of the saccade was always ipsilateral to the stimulation side. In penetrations through the paramedian vermis, the direction of the saccade changed gradually from upward oblique to downward oblique as the electrode was advanced vertically across lobule VII. The vertical component was dominant in these saccades. When the stimulation track was systematically shifted from the medial to the lateral part of the vermis, the horizontal component of the saccade increased and the vertical component decreased gradually until the saccade became almost horizontal. 5. The direction of saccades changed with the stimulus intensity; thus the topographical change in the direction of a saccade was consistent only when the stimulus intensity was adjusted to the threshold for every stimulus site. When stimulated with near threshold intensities, the evoked saccades were very seldom straight: the trajectories were curved in the responses observed at most vermal sites. The horizontal and vertical components of these saccades showed different onset latencies, durations, and peak-velocity latencies. Increasing the stimulus current produced differing effects on the two components of the saccades.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
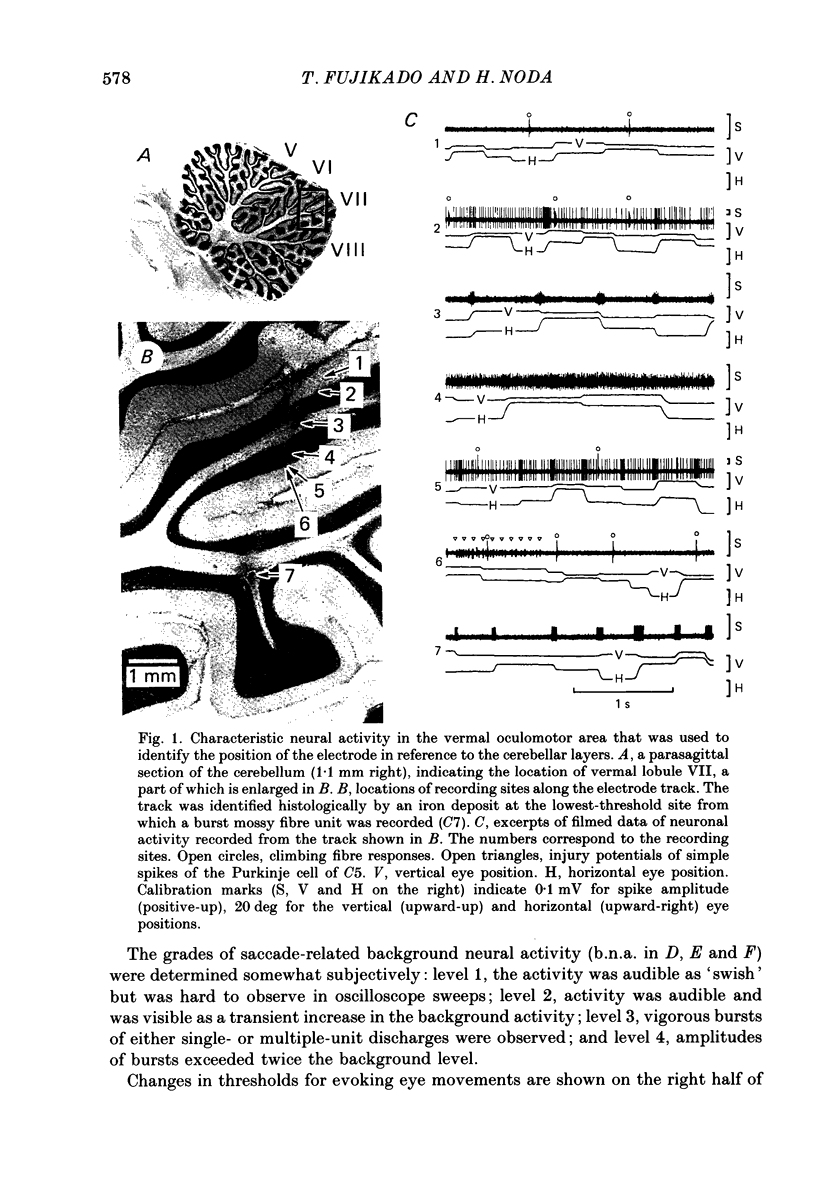
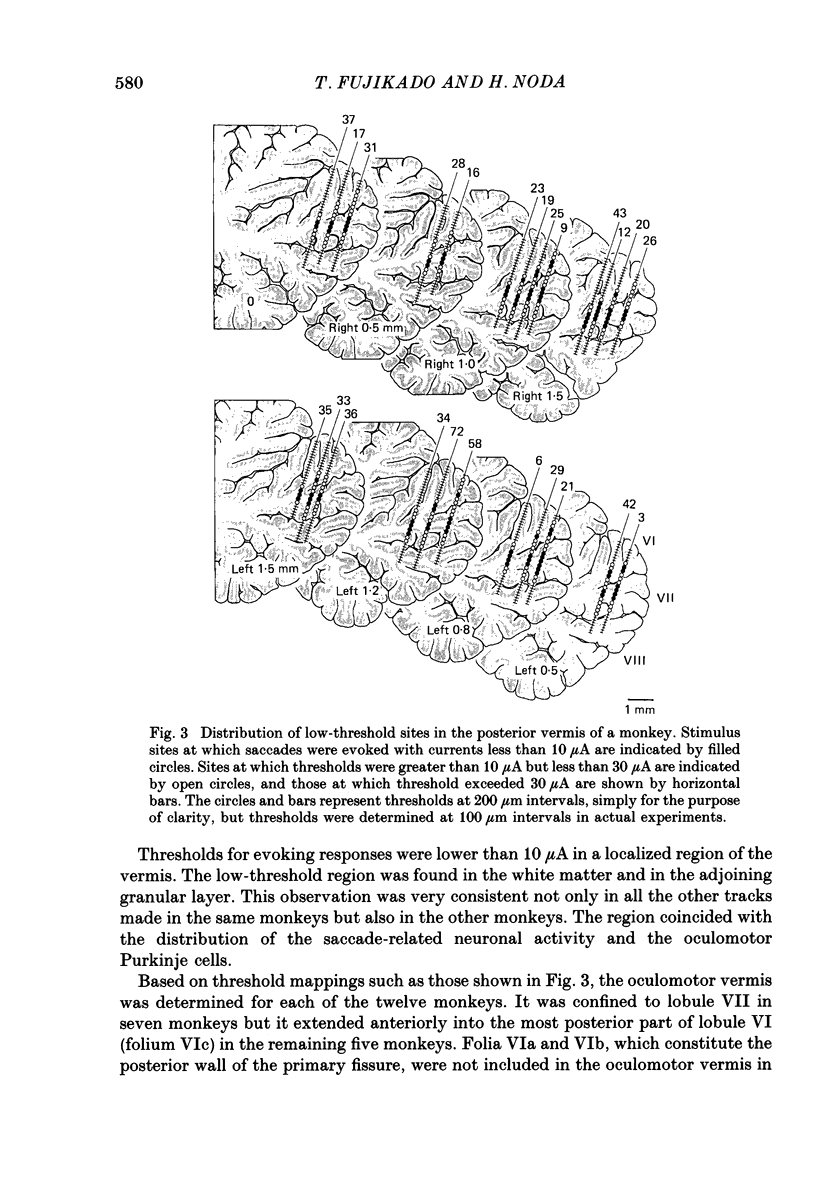
PDF
















Images in this article
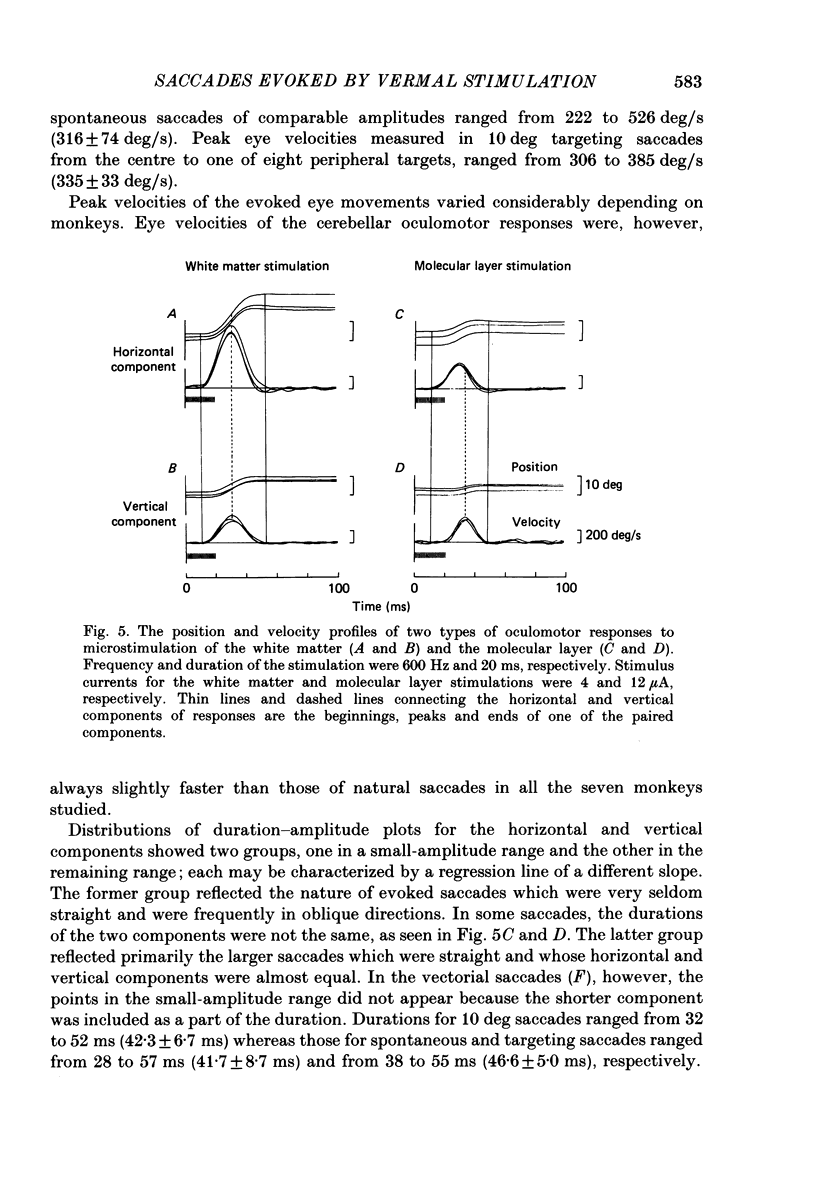
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büttner U., Büttner-Ennever J. A., Henn V. Vertical eye movement related unit activity in the rostral mesencephalic reticular formation of the alert monkey. Brain Res. 1977 Jul 15;130(2):239–252. doi: 10.1016/0006-8993(77)90273-6. [DOI] [PubMed] [Google Scholar]
- Cohen B., Goto K., Shanzer S., Weiss A. H. Eye movements induced by electric stimulation of the cerebellum in the alert cat. Exp Neurol. 1965 Oct;13(2):145–162. doi: 10.1016/0014-4886(65)90105-6. [DOI] [PubMed] [Google Scholar]
- Evinger C., Kaneko C. R., Fuchs A. F. Oblique saccadic eye movements of the cat. Exp Brain Res. 1981;41(3-4):370–379. doi: 10.1007/BF00238895. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Kaneko C. R., Scudder C. A. Brainstem control of saccadic eye movements. Annu Rev Neurosci. 1985;8:307–337. doi: 10.1146/annurev.ne.08.030185.001515. [DOI] [PubMed] [Google Scholar]
- Guitton D., Mandl G. Oblique saccades of the cat: a comparison between the durations of horizontal and vertical components. Vision Res. 1980;20(10):875–881. doi: 10.1016/0042-6989(80)90068-1. [DOI] [PubMed] [Google Scholar]
- Henn V., Cohen B. Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys. Brain Res. 1976 May 28;108(2):307–325. doi: 10.1016/0006-8993(76)90188-8. [DOI] [PubMed] [Google Scholar]
- Judge S. J., Richmond B. J., Chu F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kase M., Miller D. C., Noda H. Discharges of Purkinje cells and mossy fibres in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J Physiol. 1980 Mar;300:539–555. doi: 10.1113/jphysiol.1980.sp013178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase M., Noda H., Suzuki D. A., Miller D. C. Target velocity signals of visual tracking in vermal Purkinje cells of the monkey. Science. 1979 Aug 17;205(4407):717–720. doi: 10.1126/science.111350. [DOI] [PubMed] [Google Scholar]
- Keller E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974 Mar;37(2):316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Slakey D. P., Crandall W. F. Microstimulation of the primate cerebellar vermis during saccadic eye movements. Brain Res. 1983 Dec 12;288(1-2):131–143. doi: 10.1016/0006-8993(83)90087-2. [DOI] [PubMed] [Google Scholar]
- King W. M., Fuchs A. F. Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. J Neurophysiol. 1979 May;42(3):861–876. doi: 10.1152/jn.1979.42.3.861. [DOI] [PubMed] [Google Scholar]
- King W. M., Lisberger S. G., Fuchs A. F. Oblique saccadic eye movements of primates. J Neurophysiol. 1986 Sep;56(3):769–784. doi: 10.1152/jn.1986.56.3.769. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Luschei E. S., Fuchs A. F. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol. 1972 Jul;35(4):445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- McElligott J. G., Keller E. L. Cerebellar vermis involvement in monkey saccadic eye movements: microstimulation. Exp Neurol. 1984 Dec;86(3):543–558. doi: 10.1016/0014-4886(84)90088-8. [DOI] [PubMed] [Google Scholar]
- Noda H., Fujikado T. Involvement of Purkinje cells in evoking saccadic eye movements by microstimulation of the posterior cerebellar vermis of monkeys. J Neurophysiol. 1987 May;57(5):1247–1261. doi: 10.1152/jn.1987.57.5.1247. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972 Nov;12(11):1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Robinson D. A., Fuchs A. F. Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol. 1969 Sep;32(5):637–648. doi: 10.1152/jn.1969.32.5.637. [DOI] [PubMed] [Google Scholar]
- Ron S., Robinson D. A. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973 Nov;36(6):1004–1022. doi: 10.1152/jn.1973.36.6.1004. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1971 Sep;34(5):920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1972 Nov;35(6):915–924. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]
- Simpson J. I., Alley K. E. Visual climbing fiber input to rabbit vestibulo-cerebellum: a source of direction-specific information. Brain Res. 1974 Dec 27;82(2):302–308. doi: 10.1016/0006-8993(74)90610-6. [DOI] [PubMed] [Google Scholar]
- Suzuki D. A., Noda H., Kase M. Visual and pursuit eye movement-related activity in posterior vermis of monkey cerebellum. J Neurophysiol. 1981 Nov;46(5):1120–1139. doi: 10.1152/jn.1981.46.5.1120. [DOI] [PubMed] [Google Scholar]
- Tweed D., Vilis T. A two dimensional model for saccade generation. Biol Cybern. 1985;52(4):219–227. doi: 10.1007/BF00336978. [DOI] [PubMed] [Google Scholar]
- van Gisbergen J. A., van Opstal A. J., Schoenmakers J. J. Experimental test of two models for the generation of oblique saccades. Exp Brain Res. 1985;57(2):321–336. doi: 10.1007/BF00236538. [DOI] [PubMed] [Google Scholar]