Abstract
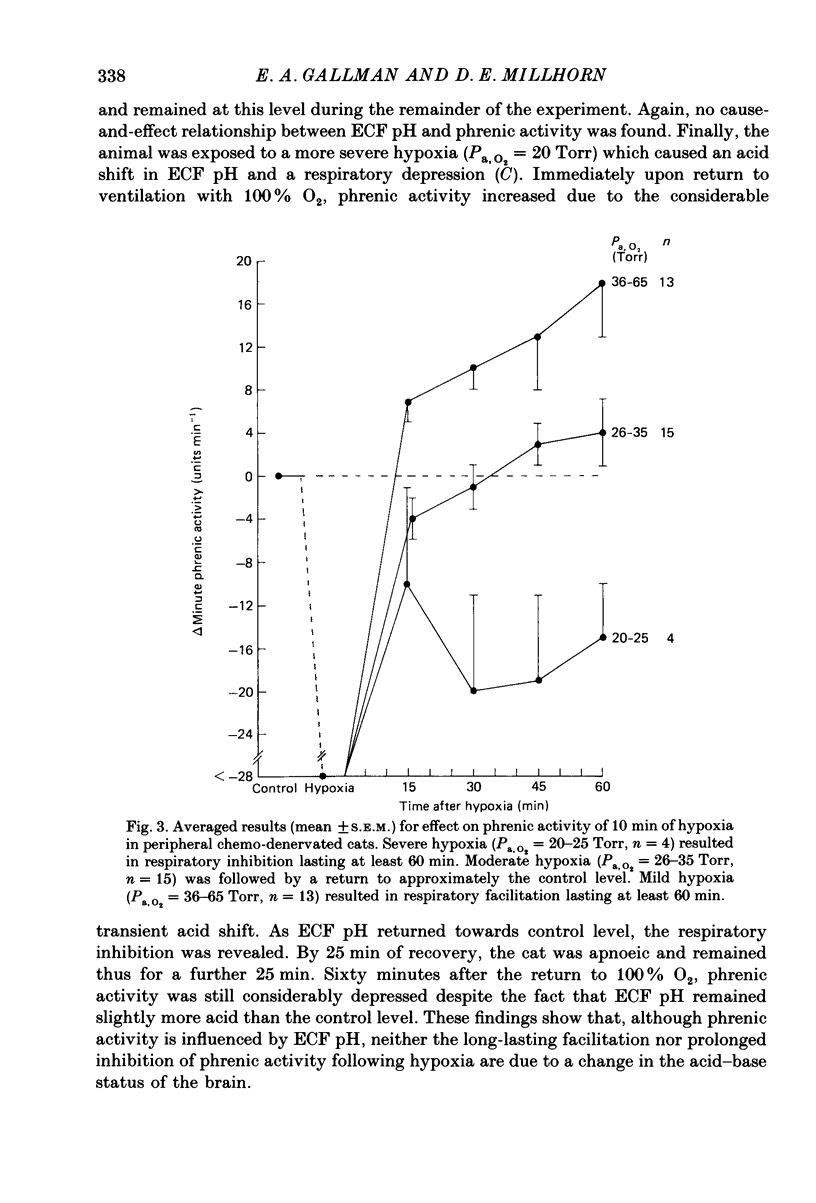
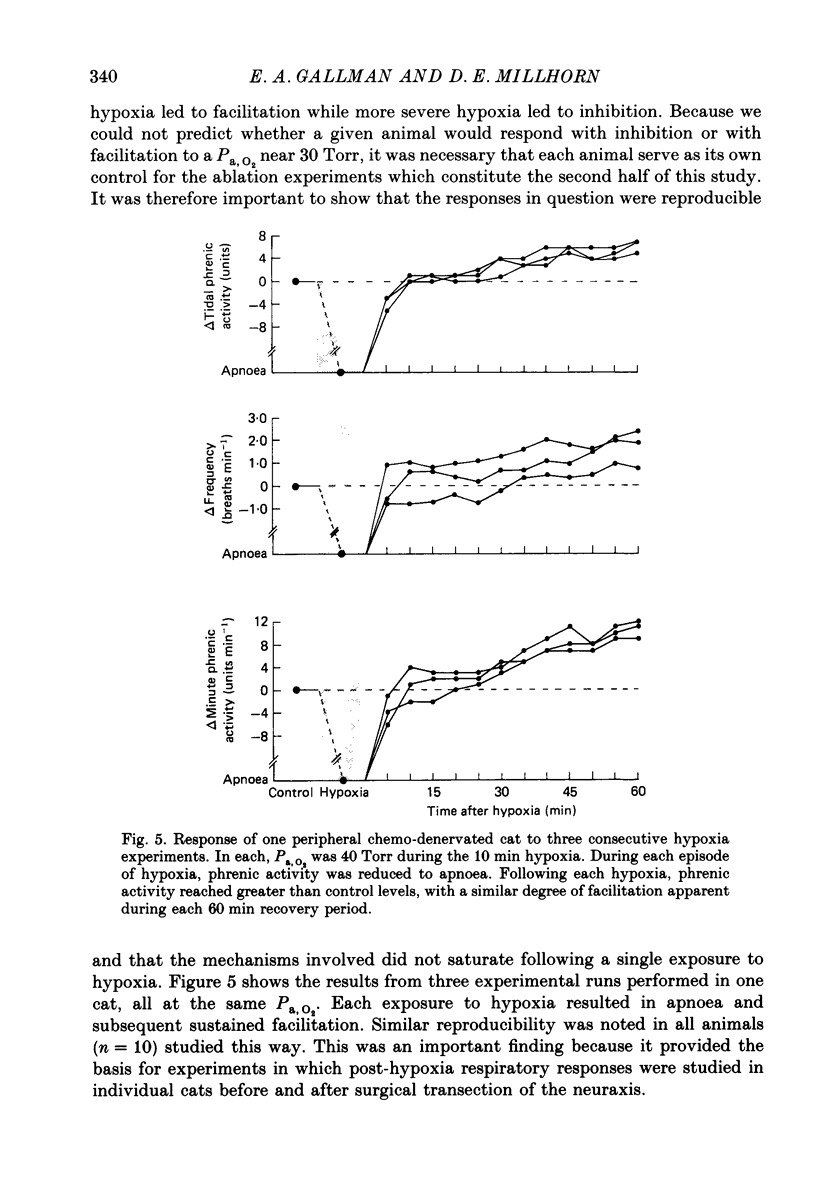
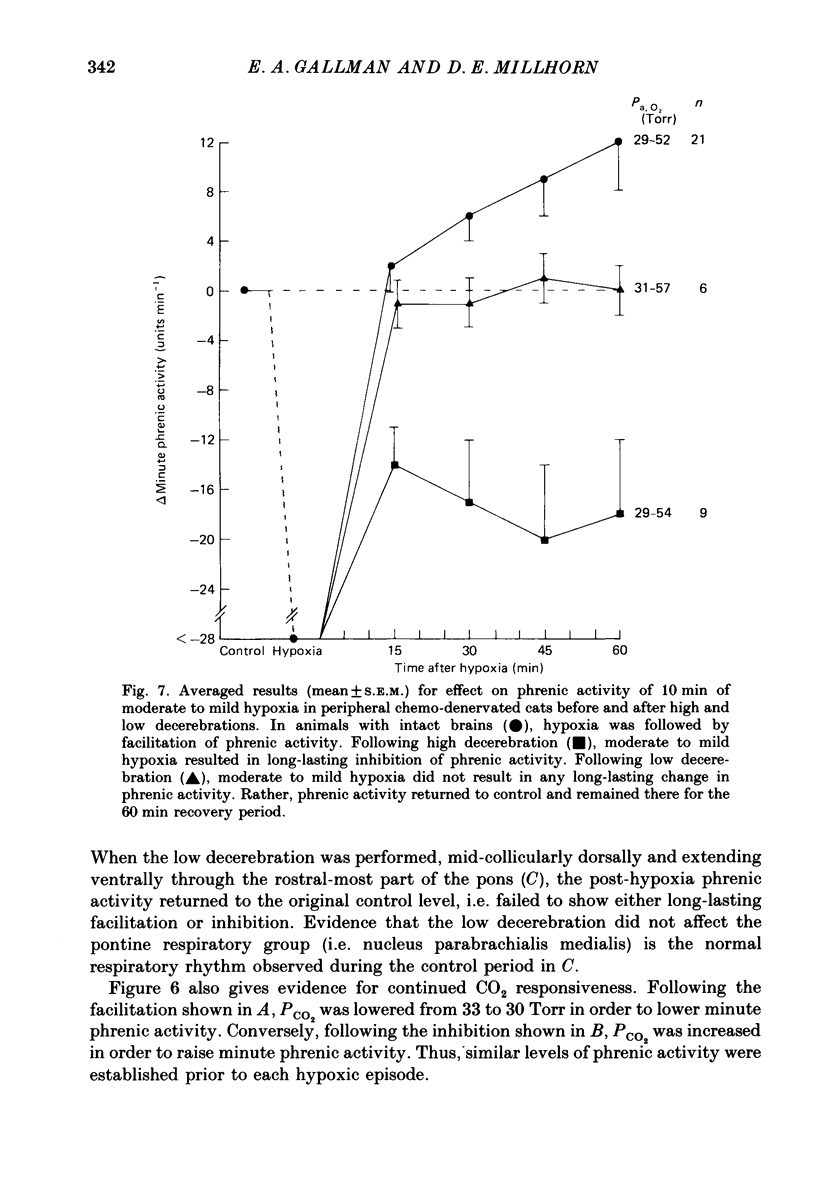
1. Central respiratory response to acute (10 min) hypoxia, as measured by phrenic nerve activity, was determined in peripheral chemo-denervated cats. 2. Hypoxia was induced by ventilating cats for 10 min at reduced inspired oxygen levels (inspired O2 fraction, FI,O2 = 0.06-0.15). The degree of hypoxaemia was determined from an arterial blood sample and ranged from 'severe' (arterial O2 pressure, Pa,O2 less than 26 Torr) to 'mild' (Pa,O2 greater than 35 Torr). The respiratory response was monitored for 1 h following return to ventilation with 100% oxygen. 3. The results confirmed the finding of prolonged (greater than 60 min) inhibition of respiration upon return to hyperoxic conditions following severe hypoxia, as reported previously (Millhorn, Eldridge, Kiley & Waldrop, 1984). A new finding was a long-lasting (greater than 60 min) facilitation of respiration following exposure to less severe (Pa,O2 greater than 35 Torr) hypoxia. 4. Medullary extracellular fluid pH was measured in six cats. Changes in pH could not explain either the prolonged inhibition following severe hypoxia or the long-lasting facilitation observed following mild hypoxia. 5. Ablation studies were performed in order to determine the locations of the neuronal substrates for the inhibitory and facilitatory mechanisms. The results of this series of experiments indicate that the mesencephalon is necessary for activation of the inhibitory mechanism, while the facilitatory mechanism requires the presence of higher brain structures, notably the diencephalon. 6. Following removal of the diencephalon, the inhibitory response was seen following even mild hypoxic insults, i.e. those shown to produce facilitation in animals with intact brains. In the absence of the mesencephalon, neither prolonged inhibition nor prolonged facilitation could be produced following hypoxia. 7. It is proposed that there are two centrally mediated long-lasting responses to acute hypoxia. Facilitation is seen following mild hypoxia. Inhibition is more likely following severe hypoxia. However, both mechanisms appear to be triggered simultaneously and the output of the central respiratory controller reflects the influence of each.
Full text
PDF




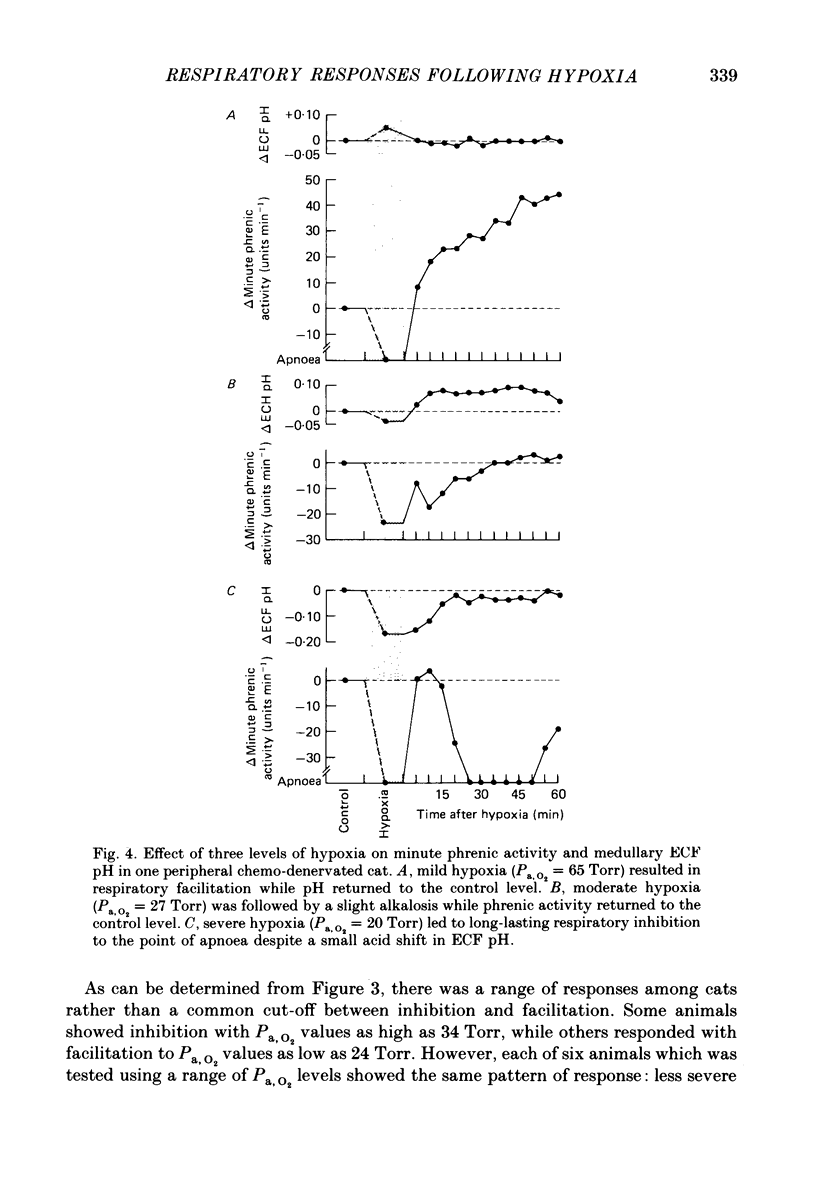






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braas K. M., Newby A. C., Wilson V. S., Snyder S. H. Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci. 1986 Jul;6(7):1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS K. W., OPPE T. E. The effect of inhalation of high and low concentrations of oxygen on the respiration of the premature infant. J Physiol. 1952 May;117(1):38–55. [PMC free article] [PubMed] [Google Scholar]
- Cherniack N. S., Edelman N. H., Lahiri S. Hypoxia and hypercapnia as respiratory stimulants and depressants. Respir Physiol. 1970;11(1):113–126. doi: 10.1016/0034-5687(70)90107-6. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Forster H. V., DoPico G. A. Ventilatory acclimatization to moderate hypoxemia in man. The role of spinal fluid (H+). J Clin Invest. 1974 Apr;53(4):1091–1100. doi: 10.1172/JCI107646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981 Feb;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Millhorn D. E., Kiley J. P., Waldrop T. G. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol. 1985 Mar;59(3):313–337. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Posthyperventilation breathing: different effects of active and passive hyperventilation. J Appl Physiol. 1973 Apr;34(4):422–430. doi: 10.1152/jappl.1973.34.4.422. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975 Oct;39(4):567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Kagawa S., Stafford M. J., Waggener T. B., Severinghaus J. W. No effect of naloxone on hypoxia-induced ventilatory depression in adults. J Appl Physiol Respir Environ Exerc Physiol. 1982 Apr;52(4):1030–1034. doi: 10.1152/jappl.1982.52.4.1030. [DOI] [PubMed] [Google Scholar]
- Kiley J. P., Eldridge F. L., Millhorn D. E. The roles of medullary extracellular and cerebrospinal fluid pH in control of respiration. Respir Physiol. 1985 Feb;59(2):117–130. doi: 10.1016/0034-5687(85)90001-5. [DOI] [PubMed] [Google Scholar]
- Kontos H. A. Regulation of the cerebral circulation. Annu Rev Physiol. 1981;43:397–407. doi: 10.1146/annurev.ph.43.030181.002145. [DOI] [PubMed] [Google Scholar]
- Kostopoulos G. K., Limacher J. J., Phillis J. W. Action of various adenine derivatives on cerebellar Purkinje cells. Brain Res. 1975 Apr 25;88(1):162–165. doi: 10.1016/0006-8993(75)90966-x. [DOI] [PubMed] [Google Scholar]
- Kostopoulos G. K., Phillis J. W. Purinergic depression of neurons in different areas of the rat brain. Exp Neurol. 1977 Jun;55(3 Pt 1):719–724. doi: 10.1016/0014-4886(77)90296-5. [DOI] [PubMed] [Google Scholar]
- LANCE J. W., ADAMS R. D. The syndrome of intention or action myoclonus as a sequel to hypoxic encephalopathy. Brain. 1963 Mar;86:111–136. doi: 10.1093/brain/86.1.111. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Milhorn H. T., Jr Central ventilatory responses to O2 and CO2 at three levels of carotid chemoreceptor stimulation. Respir Physiol. 1975 Dec;25(3):319–333. doi: 10.1016/0034-5687(75)90007-9. [DOI] [PubMed] [Google Scholar]
- Madison D., Niedermeyer E. Epileptic seizures resulting from acute cerebral anoxia. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):381–386. doi: 10.1136/jnnp.33.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Body R. L., Robson G. J., Sinclair J. D. Restoration of hypoxic respiratory responses in the awake rat after carotid body denervation by sinus nerve section. J Physiol. 1986 Nov;380:61–73. doi: 10.1113/jphysiol.1986.sp016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Tenney S. M. Hypoxia-induced tachypnea in carotid-deafferented cats. Respir Physiol. 1975 Jan;23(1):31–39. doi: 10.1016/0034-5687(75)90069-9. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Kiley J. P., Waldrop T. G. Prolonged inhibition of respiration following acute hypoxia in glomectomized cats. Respir Physiol. 1984 Sep;57(3):331–340. doi: 10.1016/0034-5687(84)90081-1. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980 Jul;41(1):87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980 Dec;42(3):171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Edstrom J. P., Kostopoulos G. K., Kirkpatrick J. R. Effects of adenosine and adenine nucleotides on synaptic transmission in the cerebral cortex. Can J Physiol Pharmacol. 1979 Nov;57(11):1289–1312. doi: 10.1139/y79-194. [DOI] [PubMed] [Google Scholar]
- Rubio R., Berne R. M., Bockman E. L., CURNISH R. R. Relationship between adenosine concentration and oxygen supply in rat brain. Am J Physiol. 1975 Jun;228(6):1896–1902. doi: 10.1152/ajplegacy.1975.228.6.1896. [DOI] [PubMed] [Google Scholar]
- Schiff S. J., Somjen G. G. Hyperexcitability following moderate hypoxia in hippocampal tissue slices. Brain Res. 1985 Jul 1;337(2):337–340. doi: 10.1016/0006-8993(85)90071-x. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Mercer R. R., Eldridge F. L. Servo control of end-tidal CO2 in paralyzed animals. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):133–136. doi: 10.1152/jappl.1978.45.1.133. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Katims J. J., Annau Z., Bruns R. F., Daly J. W. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981 May;78(5):3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney S. M., Ou L. C. Ventilatory response of decorticate and decerebrate cats to hypoxia and CO2. Respir Physiol. 1977 Feb;29(1):81–92. doi: 10.1016/0034-5687(77)90119-0. [DOI] [PubMed] [Google Scholar]
- Weil J. V., Zwillich C. W. Assessment of ventilatory response to hypoxia: methods and interpretation. Chest. 1976 Jul;70(1 Suppl):124–128. [PubMed] [Google Scholar]
- Winn H. R., Rubio R., Berne R. M. Brain adenosine concentration during hypoxia in rats. Am J Physiol. 1981 Aug;241(2):H235–H242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- Zetterström T., Vernet L., Ungerstedt U., Tossman U., Jonzon B., Fredholm B. B. Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci Lett. 1982 Apr 16;29(2):111–115. doi: 10.1016/0304-3940(82)90338-x. [DOI] [PubMed] [Google Scholar]





