Abstract
The primary aim of this study was to investigate how measurements from different regions along the rectus femoris (RF) and vastus lateralis (VL) influence muscle morphology, including muscle thickness (MT), muscle stiffness, and muscle quality. An exploratory aim was to examine whether an association exists between voluntary and involuntary force and muscle morphology across the same regions. In one session, participants (n = 13) underwent ultrasound imaging (US), followed by knee extension maximal isometric voluntary contractions and evoked contractions. US recordings (at rest) and testing were conducted while participants were seated at 90º knee flexion (dominant leg) on an isokinetic dynamometer. Muscle morphology was recorded at proximal, medial, and distal regions of RF and VL. During maximum contractions, participants were instructed to exert maximal effort as fast and as forcefully as possible for 5 s, while evoked contractions were performed via femoral nerve stimulation. A one-way repeated measures ANOVA was used for the main aim, while Spearman bivariate correlations were used for the exploratory aim. The primary findings showed that the RF and VL muscles were significantly larger in the medial region (P ≤ 0.023), with no significant differences in muscle quality or stiffness within the same muscle. Additionally, a significant overall relationship was observed between muscle quality and the rate of force development in both muscles (P ≤ 0.037). In conclusion, muscle size varies across the length of the VL and RF muscles, with no changes in muscle quality or stiffness. Furthermore, muscle quality demonstrates a significant association with rate of force development.
Keywords: Ultrasound imaging, Muscle thickness, Muscle quality, Muscle stiffness
Subject terms: Physiology, Musculoskeletal system, Skeletal muscle
Introduction
The ability to produce maximal and rapid force is partially underpinned by muscle morphology, such as muscle size1,2, muscle quality3, and muscle stiffness4,5. All these muscle characteristics are often measured using ultrasound imaging (US). US is widely used for its non-invasive nature and real-time feedback capabilities for evaluating and studying soft tissue alterations, aiding in the identification of medical conditions, and understanding muscle morphology6. A major limitation of prior studies that used US to explore the influence of muscle morphology on force production is the measurement of a single muscle region7–9. This can be an issue, particularly given the possible change of muscle morphology (i.e., muscle size) across its length10–12. Muscle size varies along its length and may differ between muscles, but this has not been extensively studied. For instance, previous research laboratories showed an overall value of muscle size for the whole muscle group rather than specific muscles (e.g., quadriceps femoris)12,13. In addition to that, most past studies have primarily focused on muscle size. Thus, there is still a lack of clarity regarding how other aspects of muscle morphology (e.g., muscle stiffness and quality) and other relevant factors (e.g., subcutaneous tissue) change at varying muscle lengths. Additionally, the relationship between these measurements and force production remains unclear.
Muscle shape can vary along its length14,15; hence, it is reasonable to assume that muscle morphology may also alter across muscle length. Indeed, researchers have shown that some muscles have different sizes across their length11,12,16. Although the knee extensors are among the most studied muscle groups in sports and rehabilitation, there is limited evidence on how muscle morphology changes along the length of this muscle group based on muscle thickness measurements from US. This is important since US is a widely used technique due to its low cost and relatively easy manipulation17. Two studies used US to assess muscle size (muscle thickness, MT) of the quadriceps femoris and reported larger muscle size at more proximal sites compared to distal12,13. However, they did not report values from the other quadricep muscles. Another study demonstrated that the vastus lateralis (VL) and rectus femoris (RF) showed variations in muscle size along their length, as measured by cross-sectional area (CSA) via magnetic resonance imaging area16. The proximal and medial regions of the VL were larger than its distal location, whereas for the RF, the distal region was larger than the medial and proximal region16. Thus, the overall representation of muscle size from a muscle group may not accurately represent what happens at each muscle. There is a possibility that different measurement techniques, such as MT using ultrasound versus CSA using magnetic resonance imaging, may impact the outcome. Nonetheless, studies that measured muscle size using US at a single site of the quadriceps femoris and used it to infer the influence on force production may yield limited results8,18–20. This is due to possible muscle changes across different sites of the same muscle. Further understanding of how MT measurements across different regions of individual muscles of the quadriceps femoris influence force production is important.
Muscle quality and stiffness are two other important characteristics of muscle morphology, given the influence they have on force production3–5. US allows for the measurement of echo intensity, which has been used as an index of muscle quality21, while shear wave elastography enables the measurement of muscle stiffness22. Although both measurements have been widely used in different contexts, it is unclear how muscle quality and stiffness change across muscle length. One study has demonstrated that muscle stiffness changes across the RF muscle length, with stiffness decreasing from proximal to distal sites23. Other than that, the majority of studies have used a single measure of US from any of the quadriceps femoris muscles to determine muscle quality and stiffness8,24–26. For this reason, it is still unclear if muscle quality and stiffness change across the muscle length and its impact on the relationship with different manifestations of force. Given previous findings23 and the observation that muscle shape changes along its length15, it is reasonable to hypothesize that muscle composition may differ within the same muscle. Given previous findings and how muscle changes the shape within its length15, it is reasonable to hypothesize that muscle content could be different in different parts within the same muscle. This is a critical point to address because it is common for researchers from different laboratories to use different anatomical references when performing ultrasound measurements. For example, one research group performed RF US recordings at 50% of the horizontal line drawn halfway between the greater trochanter of the femur and the lateral knee joint line25, whereas another group used 50% of the distance between the proximal and distal myotendinous junctions of the leg23. Assuming that muscle stiffness and/or quality varies across different regions of a muscle, inconsistent anatomical landmarks may lead to measurement discrepancies between studies. Such variability complicates the integration of findings, limiting clinicians’ and researchers’ ability to fully interpret the phenomenon and apply it in evidence-based practice.
Therefore, this study has a twofold aim. First, to investigate how measurements from different regions along the muscle length of the RF and VL influence MT, muscle stiffness, and muscle quality measurements. Given the changes in muscle shape along its length and findings from previous research, it was hypothesized that these measurements would vary across the muscle length of the RF and VL. The second and exploratory, aim is to investigate whether an association exists between voluntary (maximal force and rate of force development) and involuntary force (twitch force) and muscle morphology (MT, stiffness, and quality) across different regions of the RF and VL.
Results
Differences in muscle morphology across muscle regions
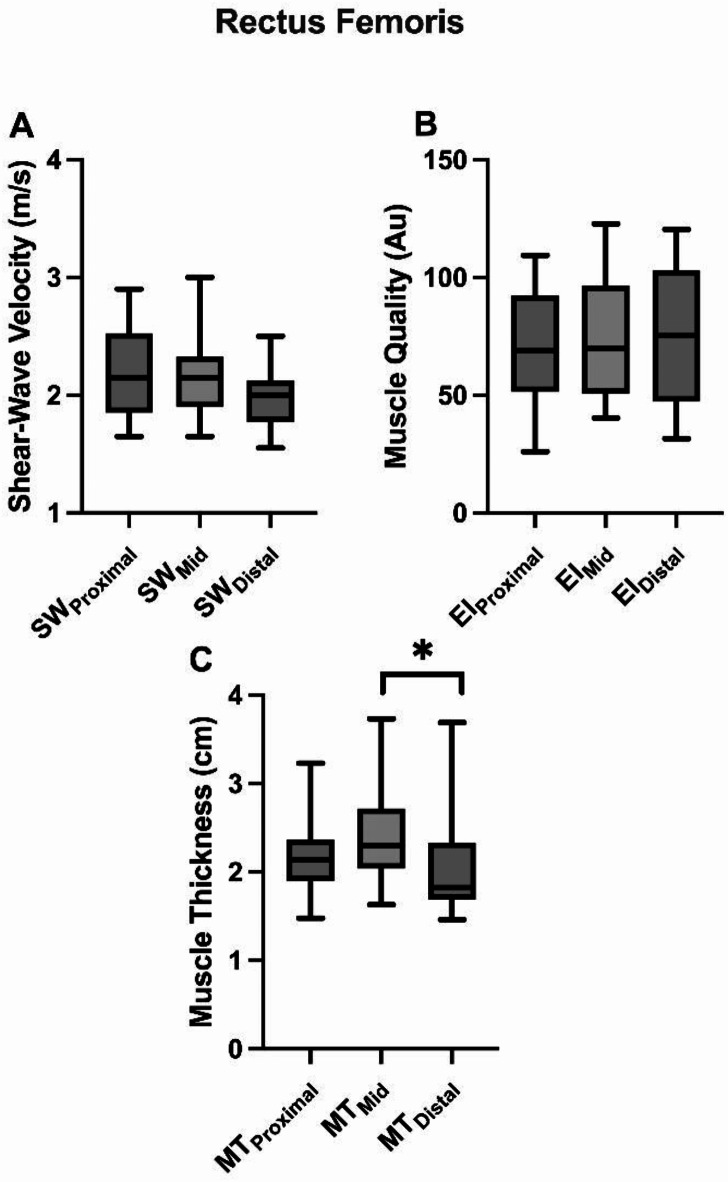
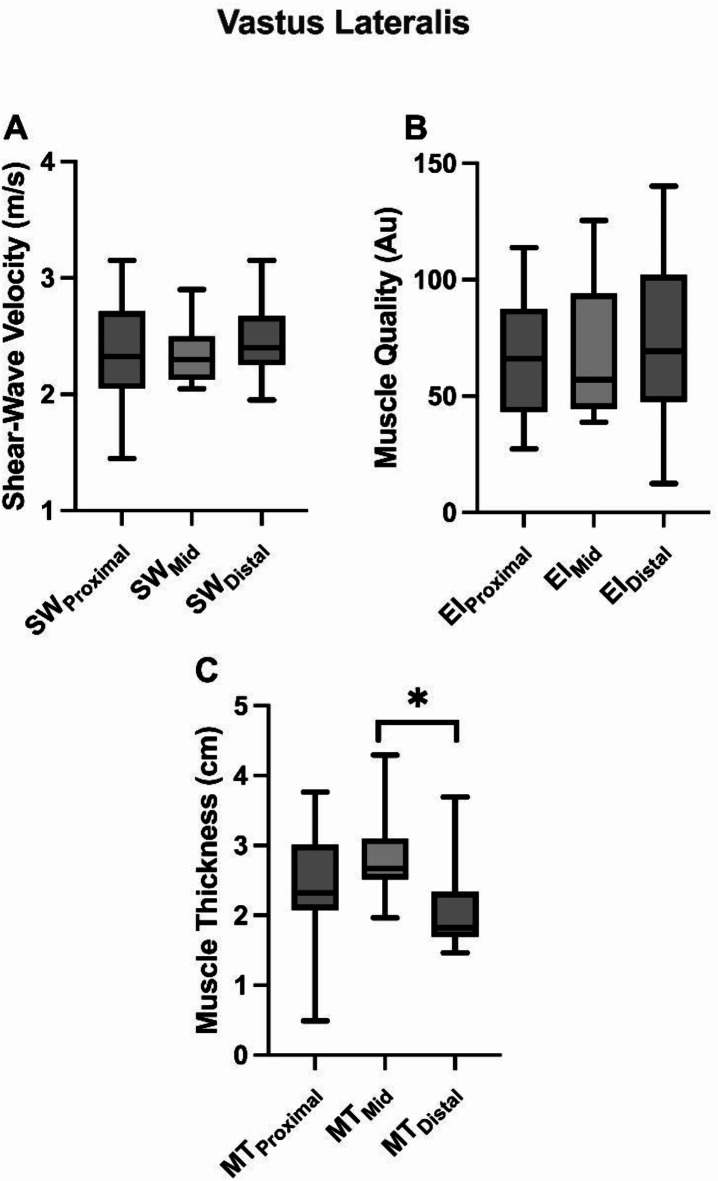
Rectus Femoris: The analysis revealed no significant differences across muscle length (proximal, medial, and distal) for muscle stiffness [F(2) = 3.199; p = 0.59; Fig. 1A] and quality [F(2) = 1.206; p = 0.317; Fig. 1B]. However, a significant difference was found between muscle size and the different locations [x² (2) = 8.769; p = 0.012; Fig. 1C], with the distal location being smaller than the medial location (Z=-2.272; p = 0.023, d = 0.52 “medium”). Vastus Lateralis: The results also revealed no statistically significant differences among the different muscle lengths when analyzing muscle stiffness [F(2) = 0.734; p = 0.492, Fig. 2A] and muscle quality [x² (2) = 2.923; p = 0.232, Fig. 2B]. Furthermore, a significant difference was observed between MT and muscle locations [x² (2) = 8.000; p = 0.018], specifically between the distal and medial positions (Z=-2.271; p = 0.023, d = 1.16 “large”), Fig. 2C.
Fig. 1.
A comparison of (A) shear-wave velocity, (B) echo intensity (muscle quality), and (C) muscle thickness between different regions of the rectus femoris muscle. A symbol (*) indicates significant differences between means (p < 0.05).
Fig. 2.
A comparison of (A) shear-wave velocity, (B) echo intensity (muscle quality), and (C) muscle thickness between different regions of the vastus lateralis muscle. A symbol (*) indicates significant differences between means (p < 0.05).
Association between force measures and muscle morphology
Rectus Femoris: No significant correlations were found between muscle stiffness at any muscle region with RFD, MF, and TF (r ≥ 0.072; p > 0.064), Table 1. However, muscle quality was significantly correlated with RFD at all muscle regions, with the lowest correlation found in the medial location (r = -0.687; p = 0.010), Table 1. Also, the mean value of muscle quality was also significantly correlated with RFD (r = -0.582; p = 0.037). MT (r ≥ 0.082; p > 0.078) and SF (r ≥ 0.104; p > 0.090) did not significantly correlate with any muscle force measures at any probe position.
Table 1.
Bivariate correlations between rate of force development (RFD), maximum force (MF), and twitch force (TF) with muscle thickness, muscle stiffness, and quality from rectus femoris muscle.
| RFD | MF | TF | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Rectus femoris | ||||||
| Muscle stiffness proximal | 0.113 | 0.713 | 0.072 | 0.816 | 0.143 | 0.640 |
| Muscle stiffness medial | 0.135 | 0.660 | 0.463 | 0.111 | 0.527 | 0.064 |
| Muscle stiffness distal | 0.416 | 0.157 | 0.427 | 0.146 | 0.201 | 0.510 |
| Muscle stiffness all | 0.184 | 0.547 | 0.303 | 0.315 | 0.369 | 0.215 |
| Muscle quality proximal | -0.637 | 0.019 | -0.313 | 0.297 | -0.055 | 0.859 |
| Muscle quality medial | -0.687 | 0.010 | -0.478 | 0.098 | -0.126 | 0.681 |
| Muscle quality distal | -0.588 | 0.035 | -0.335 | 0.263 | 0.209 | 0.494 |
| Muscle quality all | -0.582 | 0.037 | -0.418 | 0.158 | 0.050 | 0.986 |
| Muscle thickness proximal | -0.093 | 0.762 | 0.082 | 0.789 | -0.154 | 0.616 |
| Muscle thickness medial | 0.110 | 0.721 | 0.505 | 0.078 | -0.033 | 0.915 |
| Muscle thickness distal | 0.165 | 0.590 | 0.148 | 0.629 | -0.027 | 0.929 |
| Muscle thickness all | 0.220 | 0.471 | 0.258 | 0.394 | 0.022 | 0.943 |
Bold numbers indicate a significant correlation with p < 0.05. r = correlation coefficient, and p-values = level of significance.
Vastus Lateralis: No significant correlations were found between muscle stiffness at any muscle length with RFD, MF, and TF (r ≥ 0.086; p > 0.088), Table 2. Significant correlations were found between muscle quality and RFD at the medial and distal muscle locations (r = -0.791; p = 0.01; r = -0.637; p = 0.019), respectively. Similar to RF, the VL mean value of muscle quality was also significantly correlated with RFD (r = -0.659; p = 0.014). However, no correlation was found between muscle morphology and the other measurements of force (r = -0.352; p > 0.239). Additionally, both MT (r = 0.676; p = 0.011) at the medial muscle locations and SF (r = -0.571; p = 0.041) at the proximal muscle location showed significant correlations with MF. No other locations of MT and SF showed significant correlations, with MF (r ≥ 0.126, p > 0.071; r ≥ 0.176, p > 0.064; respectively), Table 2.
Table 2.
Bivariate correlations between rate of force development (RFD), maximum force (MF), and twitch force (TF) with muscle thickness, muscle stiffness, and quality from Vastus lateralis muscle.
| RFD | MF | TF | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Vastus lateralis | ||||||
| Muscle stiffness proximal | 0.182 | 0.571 | -0.056 | 0.863 | 0.123 | 0.704 |
| Muscle stiffness medial | 0.086 | 0.780 | -0.125 | 0.684 | 0.272 | 0.369 |
| Muscle stiffness distal | -0.204 | 0.505 | 0.326 | 0.276 | 0.491 | 0.088 |
| Muscle stiffness all | 0.030 | 0.921 | -0.155 | 0.613 | 0.199 | 0.514 |
| Muscle quality proximal | -0.500 | 0.820 | 0.110 | 0.721 | -0.132 | 0.668 |
| Muscle quality medial | -0.791 | 0.010 | -0.341 | 0.255 | -0.033 | 0.915 |
| Muscle quality distal | -0.610 | 0.027 | -0.352 | 0.239 | -0.104 | 0.734 |
| Muscle quality all | -0.659 | 0.014 | -0.214 | 0.482 | -0.033 | 0.915 |
| Muscle thickness proximal | -0.016 | 0.957 | 0.126 | 0.681 | 0.516 | 0.071 |
| Muscle thickness medial | -0.143 | 0.642 | 0.676 | 0.011 | 0.346 | 0.247 |
| Muscle thickness distal | 0.165 | 0.590 | 0.148 | 0.629 | -0.027 | 0.929 |
| Muscle thickness all | 0.093 | 0.762 | 0.187 | 0.541 | 0.412 | 0.162 |
Bold numbers indicate a significant correlation with p < 0.05. r = correlation coefficient, and p-values = level of significance.
Discussion
We investigated whether measurements from different sites along the VL and RF muscles affect muscle morphology. Our hypothesis was partially confirmed, as MT of both the VL and RF varied along the muscle length; however, only the VL showed differences in muscle quality across locations. Muscle stiffness did not vary across locations for either muscle. Additionally, we aimed to explore the association between force production and muscle morphology. Our findings revealed an overall significant relationship between muscle quality and RFD for both muscles. Thus, muscle quality may influence how rapid force is produced, but it may not affect the maximal capacity to produce maximal or involuntary force.
The differences in muscle size across the various regions of the VL and RF are not surprising, as both muscles exhibit changes in shape from origin to insertion15. Previous research has reported changes in muscle size (CSA) for both the RF and VL across different regions of the muscle16. The authors showed the VL had a larger size at proximal and medial locations compared to the distal location, while the RF had a larger size at a distal location than its medial and proximal locations16. In contrast, we found higher muscle size at the medial region of the VL and RF compared to the distal region, while the proximal and distal regions were similar. Although the results are somewhat different, the pattern for the VL was very similar but it was the opposite for the RF when comparing studies. The difference in results could be due to the employment of different measurement techniques and equipment, which in this case was MT using ultrasound versus CSA using magnetic resonance imaging. Another possibility is the different populations between studies; we used a mixed sample of males and females, while the previous study16 only included females. Maybe there are differences in muscle morphology between males and females.
This is the first study to demonstrate that VL or RF muscle quality and muscle stiffness do not change across its length. This appears to be opposite to previous findings showing that RF muscle stiffness decreases from proximal to distal regions23. The discrepant findings might be due to the previous investigation only reporting a descriptive difference, with no actual statistical analysis performed to compare the values between regions of the muscle. Furthermore, because we identified changes in MT, these results suggest that the relative composition of connective tissue and fat may adjust accordingly within those muscles, yet not substantially affect muscle quality or stiffness. Consequently, for muscle quality and muscle stiffness, a recording of one site might be enough to represent what is happening at the whole muscle, which is not true for MT.
Muscle stiffness was not associated with voluntary (MF and RFD) or involuntary force (TF) in the present study. Opposite, Ando and Suzuki (2019) demonstrated that maximum torque and the ability to rapidly produce torque in the plantar flexors were associated with muscle stiffness of the medial gastrocnemius4. Additionally, another study examined the relationship between VL muscle stiffness and various lower-limb performance measures—specifically, vertical jumps, countermovement jumps, rebound jumps, and multi-joint leg extension power. The author reported mixed results, with certain performance parameters (e.g., rebound jump) showing a significant correlation with muscle stiffness, while others (e.g., countermovement jump) did not27. These contradictory findings may indicate that the relationship between strength and muscle stiffness is task-dependent, as we assessed an MVC while the other author examined jump tasks and dynamic contractions. Nonetheless, previous studies have shown that voluntary force (RFD and MF) is influenced by the level of activation of the target muscles, the size of the muscles, and the quality of the muscle2,3,28,29. This information, combined with the lack of association with involuntary force, suggests that RF and VL muscle stiffness is likely less influenced by the active contractile elements responsible for force production and is primarily determined by passive structural components, such as the extracellular matrix and connective tissues30. Future studies may explore this possibility.
The ability to rapidly produce force (RFD), was influenced by muscle quality in both muscles. Echo intensity, our measure of muscle quality, evaluates the gray-scale of ultrasound images, where skeletal muscle appears hypoechogenic (closer to black), while fibrous tissue and intramuscular fat are hyperechogenic (closer to white)21. Nonetheless, there is some controversy regarding the accuracy of echo intensity in measuring fibrous tissue and intramuscular fat. Considering this information, we demonstrated that muscles with higher quality—potentially containing less fibrous tissue and intramuscular fat—are capable of producing force more quickly. This finding aligns with a previous study, which also found no significant association between muscle size and rapid torque production but showed that muscles with lower fat content produce torque at a faster rate31. Hence, individuals aiming to produce force quickly might benefit from muscles with lower fat and connective tissue content.
In contrast to previous research, overall our data set did not show that larger muscles were able to produce higher MF or involuntary force. The only exception was the VL muscle size in the medial region, which showed a significant (moderate) association with MF. In accordance with our results, we found at least one study using ultrasound measurements that did not identify a relationship between muscle size and the strength of the knee extensors32. However, other studies have demonstrated that larger quadriceps femoris muscles are often capable of producing greater knee extension forces voluntarily18,31,33,34. These discrepant findings might be due to differences in methodology between our study and others. First, the associations in our study were analyzed for a single muscle, while the force measurements reflect the combined output of all four quadriceps femoris muscles. This may partially explain the overall lack of significant relationships in our results. Secondly, we used US images from a longitudinal view and measured RF and VL muscle thickness. There has been debate about the accuracy of ultrasound imaging in estimating muscle size33,35. The use of measurements from magnetic resonance imaging and/or computed tomography scans provides a more accurate assessment of muscle size. These imaging techniques allow for the measurement of the entire muscle at small intervals across its length (e.g., 4 mm slices), enabling detailed volumetric and cross-sectional area analyses. Although our study measured three different parts of each muscle, this approach was not enough to show a significant relationship between muscle size and strength. Future studies may use different techniques to assess quadriceps femoris muscle size, and verify its influence on the ability to produce voluntary and involuntary force.
Some limitations should be highlighted here. While we had sufficient statistical power to address the primary aim of our study, we did not have adequate power for the secondary aim. Thus, the findings related to the secondary, and exploratory, aim should be interpreted with caution. Future studies should replicate this portion of the experiment with a larger sample size to confirm or refute our findings. Furthermore, our rest interval between the MVC and the evoked contraction was at least 5 minutes. Although some evidence supports this duration36, we acknowledge it may not have been sufficient for certain participants. Nonetheless, we adopted this strategy to minimize participant drop-outs due to the discomfort caused by the electrical stimulus. While our participant pool is nearly evenly split between males and females—reducing the likelihood of significant gender bias—there may still be sex-related differences in our measurements. Unfortunately, our sample size was insufficient to determine this conclusively, and future research should further investigate potential gender-based disparities. In the present study, the test was performed at 90º of knee flexion. It is well-known that the ability of the knee extensors to produce force varies across joint angles. Hence, the findings we presented may differ if testing is conducted at different joint angles. Moreover, while we used the term “muscle quality” to describe echo intensity measurements, this technique reflects general tissue composition (e.g., fat, connective tissue, and muscle fibers) but does not differentiate between these components. This limitation reduces the specificity of muscle quality assessments. Therefore, to better understand the role of fat content and connective tissue in force production, researchers may employ imaging techniques such as magnetic resonance imaging and/or computed tomography scans, which are considered the ‘gold standard’ for these assessments37,38.
In conclusion, while the RF and VL muscles appear to be larger in the central region, there is no difference in muscle quality and stiffness within the different parts of the same muscles. Furthermore, although a significant overall relationship was found between muscle quality and the ability to produce force quickly in both muscles, neither muscle size nor stiffness influenced any of the force measurements performed.
Methods
Participants
Thirteen individuals (ages 18 to 35 years, Table 3) with no history of muscular disorders, lower extremity surgery, or serious injuries volunteered for this study. A total sample size of 5 participants was determined by using GPower (version 3.0.1, Germany) based on a test using an analysis of variance (ANOVA repeated-measures within factor), with an alpha level of 0.05, power of 0.80; effect size (ES) of 0.71 (based on the average effect size value among the three comparisons of muscle stiffness from previous study23), number of groups, 1; number of measurements, 3; correlation among measures of 0.5; and a nonsphericity correction of 1. All volunteers provided written informed consent before their participation, and approval was granted by the University’s Institutional Review Board. The experiment was conducted in accordance with Declaration of Helsinki.
Table 3.
Characteristics of individuals are expressed as mean ± standard deviation.
| n | 13 |
|---|---|
| Females | 7 |
| Age (years) | 27.69 ± 2.49 |
| Height (m) | 1.68 ± 0.80 |
| Body mass (Kg) | 69.0 ± 21.1 |
| BMI (Kg/m2) | 24.1 ± 7.0 |
| Maximum Force (N) | 792 ± 392 |
| Twitch Force (N) | 115 ± 91.4 |
| Rate of Force Development (N/s) | 424 ± 279 |
Overview
In one session, participants were subjected to ultrasound imaging, followed by knee extension maximal isometric voluntary contractions (MVIC), and evoked contractions. Testing occurred while participants were seated on an isokinetic dynamometer (Humac Norm, Stoughton, MA). All measurements were taken on the dominant leg. The leg dominance was defined by asking the following question: which foot would you kick a ball39?
Measures and procedures
Initially, participants signed the consent form, and then their height and weight were measured.
Ultrasound measures
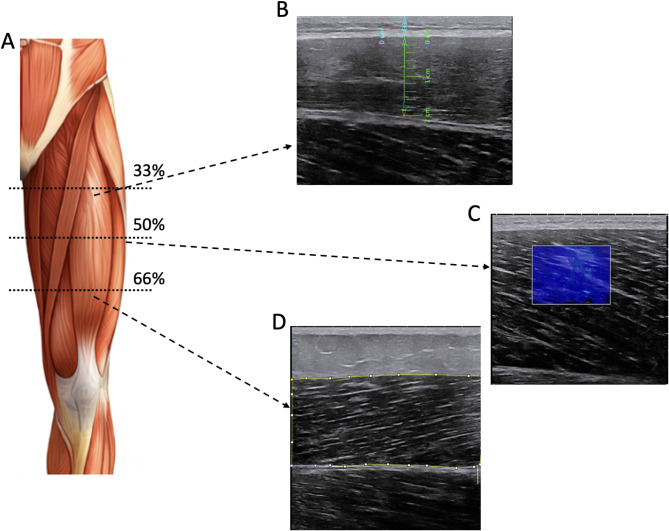
During 10 min, participants remained seated (knees at 90º of flexion, and hips at 100º) before ultrasound measurements. While they were seated, the anterior regions of the dominant thigh were marked to identify the reference points for the ultrasound image acquisition. Skin marks were made on the VL and RF muscles at 33% (distal), 50% (medial), and 66% (distal) of the distance from the anterior superior iliac spine to the lateral side of the patella40. Following skin marking, an evaluator (MBL) acquired all images. Two ultrasound images per site were obtained using B-mode ultrasonography (Aixplorer, Supersonic Imagine, Aix-en-Provence, France) with a SuperLinear 5–18 MHz frequency probe (5.5 cm). For all measurements, the probe was positioned longitudinally on both muscles (Fig. 3). Using the same ultrasound and probe, two additional images were acquired using shear-wave elastography mode (for muscle stiffness calculation) by using the General preset (SWE optimization: superficial, persistence: High, smoothing level: 5). An example of US images can be seen in Fig. 3.
Fig. 3.
Examples of B-mode and shear-wave ultrasound images from different participants. (A) A schematic representation of the distances used to record images. (B) An example of a rectus femoris image recorded at the proximal region (33% of thigh length). The blue line represents the measurement of subcutaneous fat, and the green line indicates muscle thickness. (C) An example of a vastus lateralis image recorded at the mid-thigh (50% of thigh length) using shear wave mode to measure muscle stiffness. (D) An example of a rectus femoris image recorded at the distal region (66% of thigh length). The yellow line in the image indicates the region used to calculate gray-scale values for estimating muscle quality. The image of the leg (A) was generated using the DALL-E program.
Knee extensors MVIC
Participants were securely strapped at the trunk, hips, and leg to the isokinetic machine (Humac Norm, USA) to minimize extraneous bodily movement, and performed a warm-up. The knee was positioned at 90º of knee flexion, and hips at 100º. All tasks were isometric. The warm-up consisted of a series of submaximum contractions performed at 50% (×3), 75% (×2), and 90% (×1) of perceived maximum as reported somewhere41. For the MVIC, participants were instructed to perform a maximum effort as fast and as hard as possible for 5 s, with at least 30 s of rest between each attempt. To optimize participant performance, continuous biofeedback was supplied by positioning the dynamometer’s force production display in the participant’s view. Additionally, verbal motivation was consistently provided throughout each trial.
Evoke contractions
After completing the MVIC, participants were given a minimum rest period of 5 min before the evoked twitch responses were elicited36. This time course was dected as the point Transcutaneous femoral nerve stimulation was delivered with a constant current variable voltage stimulator (DS7AH, Digitimer Ltd., Welwyn Garden City, UK) while the participant remained passive41. After applying the conductive gel, a carbon rubber anode, sized 60 × 50 mm, was placed over the greater trochanter, and a carbon rubber cathode, measuring 35 × 25 mm, was aligned with the femoral nerve within the femoral triangle. A sequential repositioning of the cathode was carried out to ensure the site of the strongest twitch response; after this identification, the cathode was taped down. After that, incremental single-pulse stimuli with 200 µs square-wave pulses were delivered (every 15 s, in 20 mA increments) until the peak twitch force plateaued. Once the plateau was reached, two additional increments were administered to confirm that the plateau level had been achieved. Subsequently, three supra-maximal stimuli were delivered (with 15 s between each stimulus) at a current of 120% of the plateau level to measure the supra-maximal twitch force (TF).
Data analysis
Ultrasound imaging
For all ultrasound measurements detailed subsequently, the measurements were performed twice for each percentage distance on each image. This process was applied to both images taken, and the average value of the measurements was used for statistical analysis. This method was consistently employed for all percentage distances at which images were acquired for all the following variables. Echo intensity (muscle quality): the ImageJ software, version 1.53a (National Institutes of Health, USA) was used for image analysis. Ultrasound images were exported from the ultrasound machine and imported to ImageJ in jpeg format. The region of interest of RF and VL was measured using the polygon tool by the same evaluator (SL) on both acquired images for all the distances. To maintain uniformity and precision in the analysis of echo intensity21, the images were displayed with identical settings for all participants, which included a gain of 62 dB, a depth of 6.0 cm, and a frequency of 15 Hz. The Intraclass Correlation (ICC(2,1)) for muscle quality between the images for each muscle and location were excellent, ranging from 0.88 to 0.92. Muscle thickness (MT): the open-source Tracker software (version 6.13, physlets.org/tracker) was utilized for image analysis39. In summary, the ultrasound images were transferred in JPEG format from the imaging device to the dedicated software. Measurements of MT were consistently conducted from these images by the same evaluator (NF). For the RF and VL muscles, MT involved determining the distance between the upper and lower aponeuroses at each image. The ICC(2,1) between the images for muscle thickness ranged from a minimum of 0.92 up to 0.97. Muscle Stiffness: after images were recorded, data analysis was performed using the ultrasound machine software by the same evaluator (RB). A circular region of interest of 10 mm in diameter was used to cover the muscles (VL and RF) and record the shear wave velocity (m/s)42. The ICC(2,1) between the images for muscle stiffness ranged from minimum of 0.78 up to 0.92. For all variables presented here, a mean value of all two sites was used for further analysis.
Maximum voluntary isometric contraction (MVIC), rate of force development (RFD), and twitch force (TF)
Force signals from the isokinetic dynamometer were synced with Spike 2 software (CED, Cambridge, UK) for analysis. The force signal underwent low-pass filtering at a threshold of 500 Hz using a fourth-order zero-lag Butterworth digital filter, and to eliminate main frequency interference, a notch filter at 50 Hz with an infinite impulse response digital filter, featuring a quality factor of 10, was applied. Maximum force (MF) was calculated as the highest instantaneous point during the MVIC. Similarly, TF was calculated as the highest instantaneous point during the evoked contraction. RFD was measured from the onset of force to the peak force, and the value was then divided by the duration of the epoch. The onset of force was identified as previously recommended43.
Statistical analysis
The statistical analyses were performed using SPSS version 29.0 (IBM Inc., Chicago, IL, USA). The level of significance was set at the α < 0.05, and data is presented as mean ± SD. It is important to highlight that echo intensity values have been reported to be influenced by subcutaneous fat21. Taking this into consideration, we tested the association between echo intensity and subcutaneous fat measurements in our sample, and there was no significant association between the two variables. Thus, no correction was made, and statistical analysis was performed using raw echo intensity. Data were first examined for normality (Shapiro-Wilk test) and homogeneity of variance (Levene’s test). For the study’s first aim, which was to analyze the differences between sites for muscle stiffness, muscle quality, MT, and SF, a one-way repeated measures ANOVA was used for each dependent variable. A Bonferroni-adjusted post-hoc test was employed when the main effect was detected in the ANOVA. Alternatively, non-normally distributed data were analyzed using the Friedman test and the Wilcoxon signed-rank test adjusted as the post hoc procedure. Cohen’s d effect sizes (d) were used to assess the magnitude of any effect and interpreted as follows: trivial, < 0.2; small, 0.2 to 0.49; medium, 0.50 to 0.79; and large, > 0.844. For the study’s second aim, which was to explore the association between measures of force production (MF, RFD, and TF) and muscle morphology (MT, muscle stiffness, and muscle quality), Spearman’s rho correlation test was used.
Author contributions
M.B.L., L.Q.Z., and V.L.G. conceived and designed research. M.L., S.L., N.F., R.B. conducted experiments. M.L., S.L., N.F., R.B., L.C.M. analyzed and processed data. All authors contribute to manuscript writing. All authors read and approved the manuscript.
Data availability
The datasets generated and/or analyzed in the current study are available upon reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Evangelidis, P. E., Massey, G. J., Pain, M. T. G. & Folland, J. P. Strength and size relationships of the quadriceps and hamstrings with special reference to reciprocal muscle balance. Eur. J. Appl. Physiol.116, 593–600 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Maden-Wilkinson, T. M., Balshaw, T. G., Massey, G. J. & Folland, J. P. Muscle architecture and morphology as determinants of explosive strength. Eur. J. Appl. Physiol.121, 1099–1110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuschel, L. B., Sonnenburg, D. & Engel, T. Factors of muscle quality and determinants of muscle strength: A systematic literature review. Healthcare10, 1937 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ando, R. & Suzuki, Y. Positive relationship between passive muscle stiffness and rapid force production. Hum. Mov. Sci.66, 285–291 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Muraki, S., Fukumoto, K. & Fukuda, O. Prediction of the muscle strength by the muscle thickness and hardness using ultrasound muscle hardness meter. SpringerPlus2, 457 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells, P. N. T. Ultrasound imaging. Phys. Med. Biol.51, R83–R98 (2006). [DOI] [PubMed] [Google Scholar]
- 7.De França, H. S. et al. The effects of adding single-joint exercises to a multi-joint exercise resistance training program on upper body muscle strength and size in trained men. Appl. Physiol. Nutr. Metab.40, 822–826 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Cadore, E. L. et al. Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men. Exp. Gerontol.47, 473–478 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Gentil, P. et al. Effect of adding single-joint exercises to a multi-joint exercise resistance-training program on strength and hypertrophy in untrained subjects. Appl. Physiol. Nutr. Metab.38, 341–344 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Diniz, R. C. R. et al. Does the muscle action duration induce different regional muscle hypertrophy in matched resistance training protocols? J. Strength. Conditioning Res.36, 2371–2380 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Pedrosa, G. F. et al. Training in the initial range of motion promotes greater muscle adaptations than at final in the arm curl. Sports11, 39 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa, B. D. D. V. et al. Does performing different resistance exercises for the same muscle group induce non-homogeneous hypertrophy?? Int. J. Sports Med.42, 803–811 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Abe, T., DeHoyos, D. V., Pollock, M. L. & Garzarella, L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur. J. Appl. Physiol.81, 0174 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Lanza, M. B. et al. Pectoralis major and triceps brachii cross-sectional area measured on different planes: the effect on the muscle size-strength relationship. J. Sports Med. Phys. Fit.63, (2023). [DOI] [PubMed]
- 15.Muscolino, J. E. Kinesiology: The Skeletal System and Muscle Function (Elsevier Inc, 2017).
- 16.Diniz, R. C. R. et al. Does the muscle action duration induce different regional muscle hypertrophy in matched resistance training protocols? J. trength Condit. Res. (2020). [DOI] [PubMed]
- 17.Bemben, M. G. Use of diagnostic ultrasound for assessing muscle size. J. Strength. Cond Res.16, 103–108 (2002). [PubMed] [Google Scholar]
- 18.Strasser, E. M., Draskovits, T., Praschak, M., Quittan, M. & Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. AGE35, 2377–2388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukumoto, Y. et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur. J. Appl. Physiol.112, 1519–1525 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Freilich, R. J., Kirsner, R. L. G. & Byrne, E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul. Disord.5, 415–422 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Stock, M. S. & Thompson, B. J. Echo intensity as an indicator of skeletal muscle quality: applications, methodology, and future directions. Eur. J. Appl. Physiol.121, 369–380 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. S. M., Gaebler-Spira, D., Zhang, L. Q., Rymer, W. Z. & Steele, K. M. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin. Biomech.31, 20–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfuraih, A. M. et al. An investigation into the variability between different shear wave elastography systems in muscle. Med. Ultrason.19, 392 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Alfuraih, A. M., Tan, A. L., O’Connor, P., Emery, P. & Wakefield, R. J. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res.31, 1755–1763 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scafoglieri, A. et al. Skeletal muscle echo intensity values differ significantly across ultrasound parameter settings. Life14, 291 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jajtner, A. R. et al. Performance and muscle architecture comparisons between starters and nonstarters in National collegiate athletic association division I women’s soccer. J. Strength. Cond. Res.27, 2355–2365 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Ema, R. Association between elastography-assessed muscle mechanical properties and high‐speed dynamic performance. Eur. J. Sport Sci.23, 1233–1239 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Dideriksen, J. L., Vecchio, D., Farina, D. & A. & Neural and muscular determinants of maximal rate of force development. J. Neurophysiol.123, 149–157 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Fitts, R. H., McDonald, K. S. & Schluter, J. M. The determinants of skeletal muscle force and power: Their adaptability with changes in activity pattern. J. Biomech.24, 111–122 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Raghavan, P., Stecco, A., Menon, R., Cowman, M. K. & Regatte, R. Mechanisms of development of passive mechanical muscle stiffness. in Spasticity and Muscle Stiffness (ed Raghavan, P.) 81–105 (Springer International Publishing, Cham, doi:10.1007/978-3-030-96900-4_6. (2022). [Google Scholar]
- 31.Frank-Wilson, A. W. et al. Associations of quadriceps torque properties with muscle size, attenuation, and intramuscular adipose tissue in older adults. Journals Gerontology: Ser. A73, 931–938 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dons, B., Bollerup, K., Bonde-Petersen, F. & Hancke, S. The effect of weight-lifting exercise related to muscle fiber composition and muscle cross-sectional area in humans. Eur. J. Appl. Physiol.40, 95–106 (1979). [DOI] [PubMed] [Google Scholar]
- 33.Balshaw, T. G., Maden-Wilkinson, T. M., Massey, G. J. & Folland, J. P. The human muscle size and strength relationship: effects of architecture, muscle force, and measurement location. Med. Sci. Sports Exerc.53, 2140–2151 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Massey, G., Evangelidis, P. & Folland, J. Influence of contractile force on the architecture and morphology of the quadriceps femoris. Exp. Physiol.100, 1342–1351 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Balshaw, T. G., Massey, G. J., Maden-Wilkinson, T. M. & Folland, J. P. Muscle size and strength: Debunking the completely separate phenomena suggestion. Eur. J. Appl. Physiol.117, 1275–1276 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Hamada, T., Sale, D. G., MacDougall, J. D. & Tarnopolsky, M. A. Postactivation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. J. Appli. Physiol. (Bethesda, Md.)88, 2131–2137 (2000). (1985). [DOI] [PubMed]
- 37.Molwitz, I. et al. Skeletal muscle fat quantification by dual-energy computed tomography in comparison with 3T MR imaging. Eur. Radiol.31, 7529–7539 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kodama, Y. et al. Comparison of CT methods for determining the fat content of the liver. Am. J. Roentgenol.188, 1307–1312 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Lanza, M. B., Rock, K., Marchese, V., Gray, V. L. & Addison, O. Ultrasound measures of muscle thickness and subcutaneous tissue from the hip abductors: Inter- and intra-rater reliability. Musculoskelet. Sci. Pract.62, 102612 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritsche, P. et al. Quadriceps muscle geometry and strength throughout maturation in National-Level male soccer players: A cross-sectional study. OAJSM Volume. 15, 159–170 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanza, M. B., Balshaw, T. G. & Folland, J. P. Is the joint-angle specificity of isometric resistance training real? And if so, does it have a neural basis? Eur. J. Appl. Physiol.119, 2465–2476 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Kot, B. C. W., Zhang, Z. J., Lee, A. W. C., Leung, V. Y. F. & Fu, S. N. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: Variations with different technical settings. PLoS ONE. 7, e44348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillin, N. A., Pain, M. T. G. & Folland, J. P. Identification of contraction onset during explosive contractions. Response to Thompson et al. ‘Consistency of rapid muscle force characteristics: influence of muscle contraction onset detection methodology’ [J electromyogr kinesiol 2012;22(6):893–900]. J. Electromyogr. Kinesiol.23, 991–994 (2013). [DOI] [PubMed]
- 44.Cohen, J. Statistical Power Analysis for the Behavioral Sciencesvol. 26 588 (Elsevier, 2006).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in the current study are available upon reasonable request from the corresponding author.