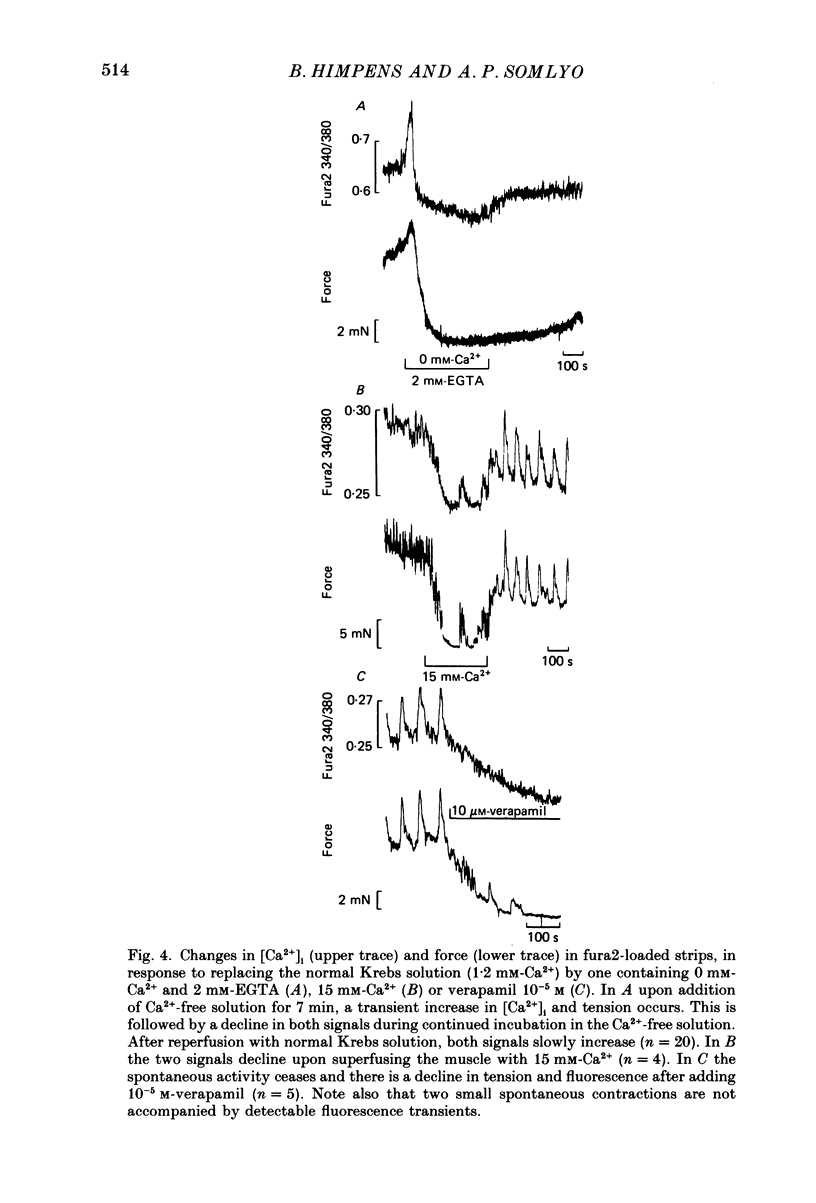
Abstract
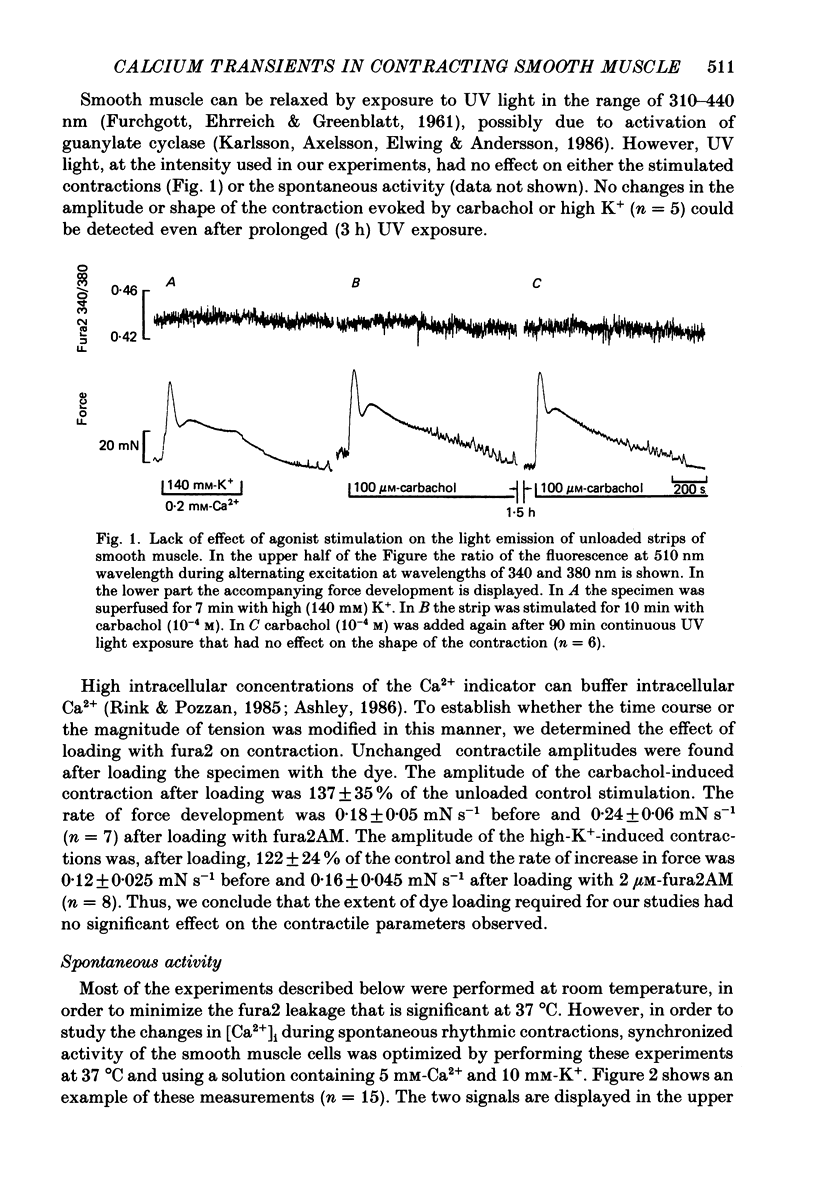
1. Fura2 was loaded by permeation and hydrolysis of the acetoxymethyl ester into smooth muscle cells of intact thin sheets of the longitudinal layer of the small intestine of the guinea-pig, to record Ca2+ transients during contraction. 2. Cytoplasmic Ca2+ ([Ca2+]i) was monitored by computing the ratio of the fluorescence signal excited at 340 and 380 nm wavelengths. The dye loading and the exposure to UV light required for the experiments had no significant effect on the contractile parameters observed. 3. Spontaneous, rhythmic increases in [Ca2+]i were often observed, preceding the onset of force. Removal of extracellular Ca2+ caused a very transient increase in [Ca2+]i accompanied by a phasic force transient; this was followed by a decline in [Ca2+]i and tension below control levels. Elevated Ca2+ from 1.2 to 15 mM also caused a fall in [Ca2+]i and a relaxation of basal tension. 4. Elevation of [K+]o increased [Ca2+]i. Graded concentrations of K+ caused graded changes in both fluorescence ratio and tension. 5. Carbachol evoked a transient increase in [Ca2+]i and contraction. Thereafter, in spite of the continued presence of the drug, both signals declined, presumably as the result of cholinergic desensitization. The initial phasic force response to carbachol was usually followed by an 'after-contraction', that was only occasionally accompanied by a similar (small) secondary rise in the fluorescence signal. 6. In depolarized smooth muscle, both in the presence and in the absence of extracellular Ca2+, carbachol induced a transient increase in [Ca2+]i, indicating that Ca2+ release from intracellular stores is a major mechanism of pharmacomechanical coupling. 7. In some preparations an applied stretch caused, after a few seconds, a rise in [Ca2+]i and force development.
Full text
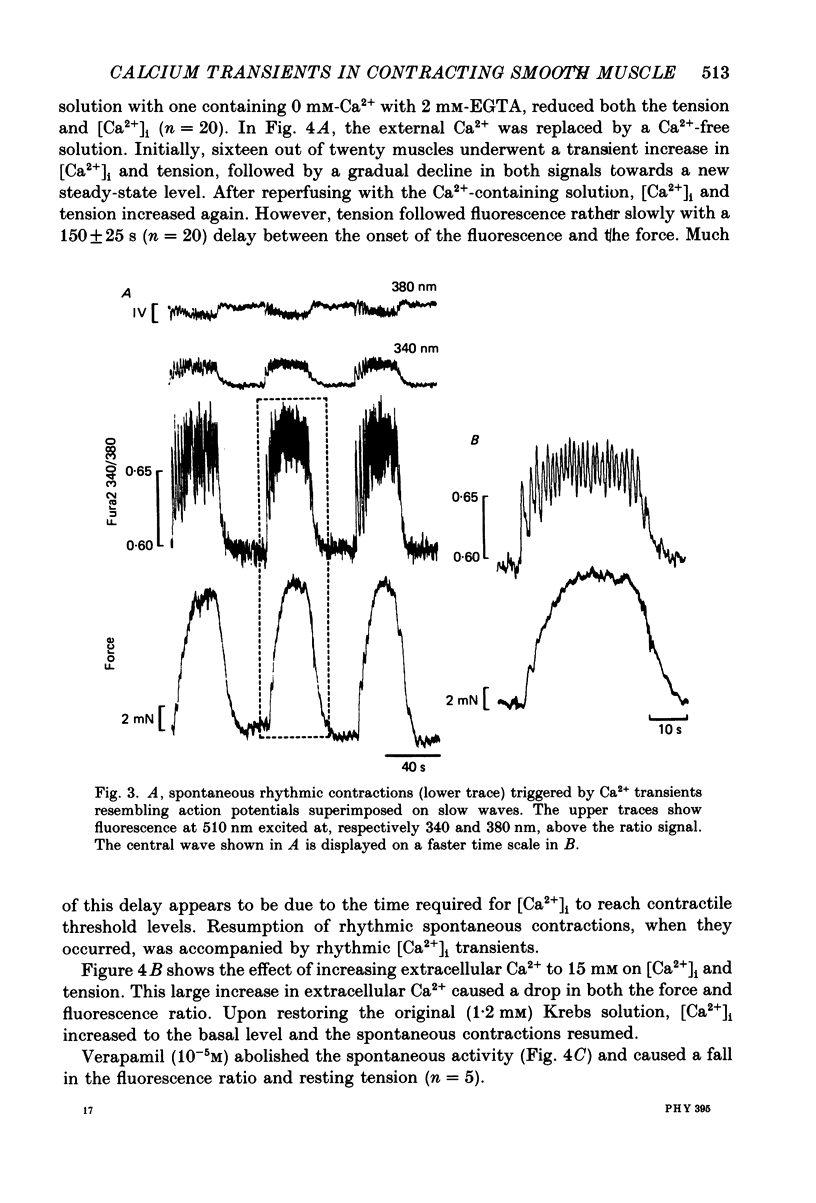
PDF



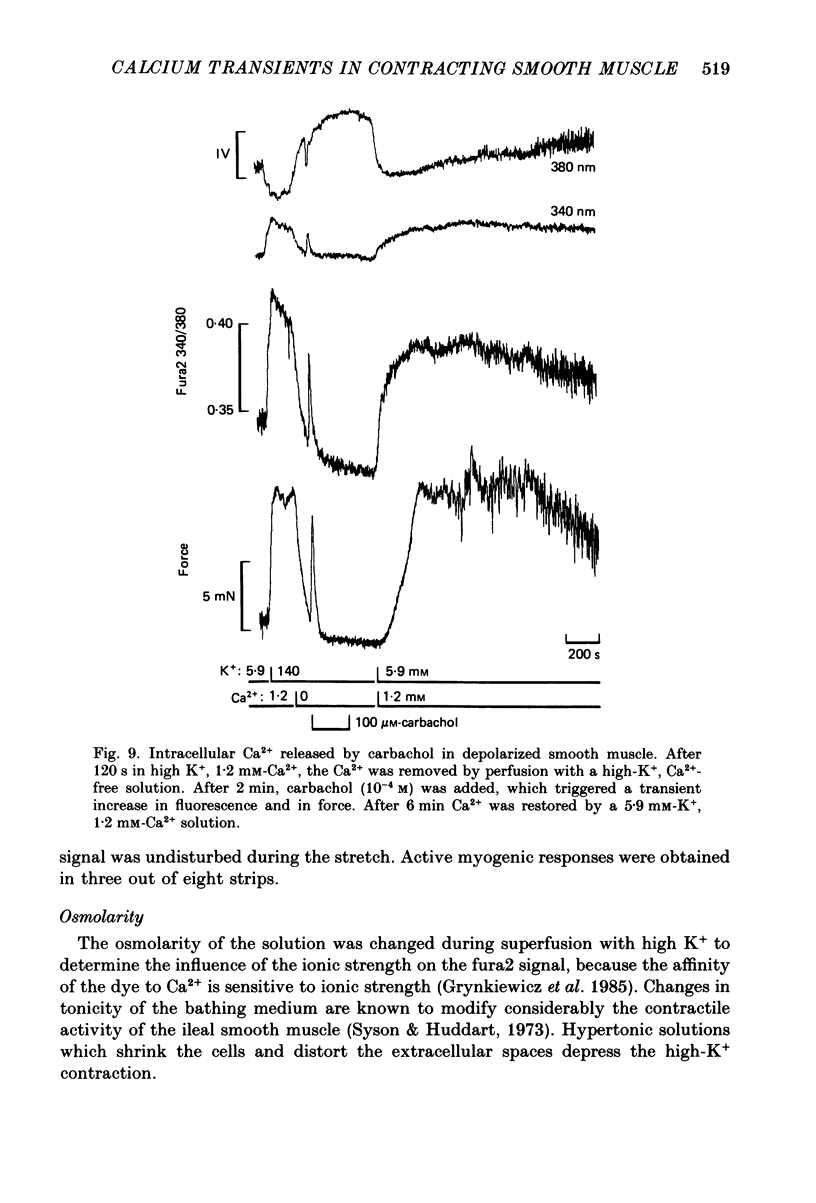
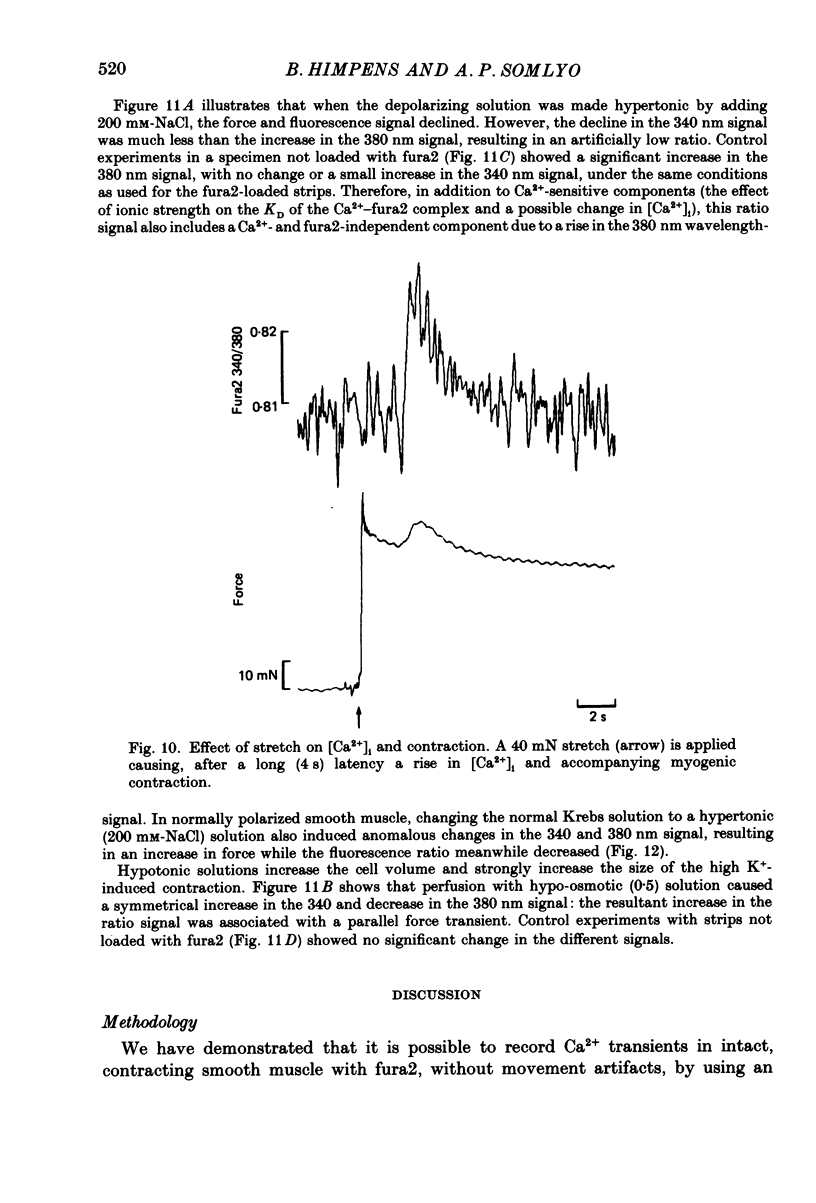
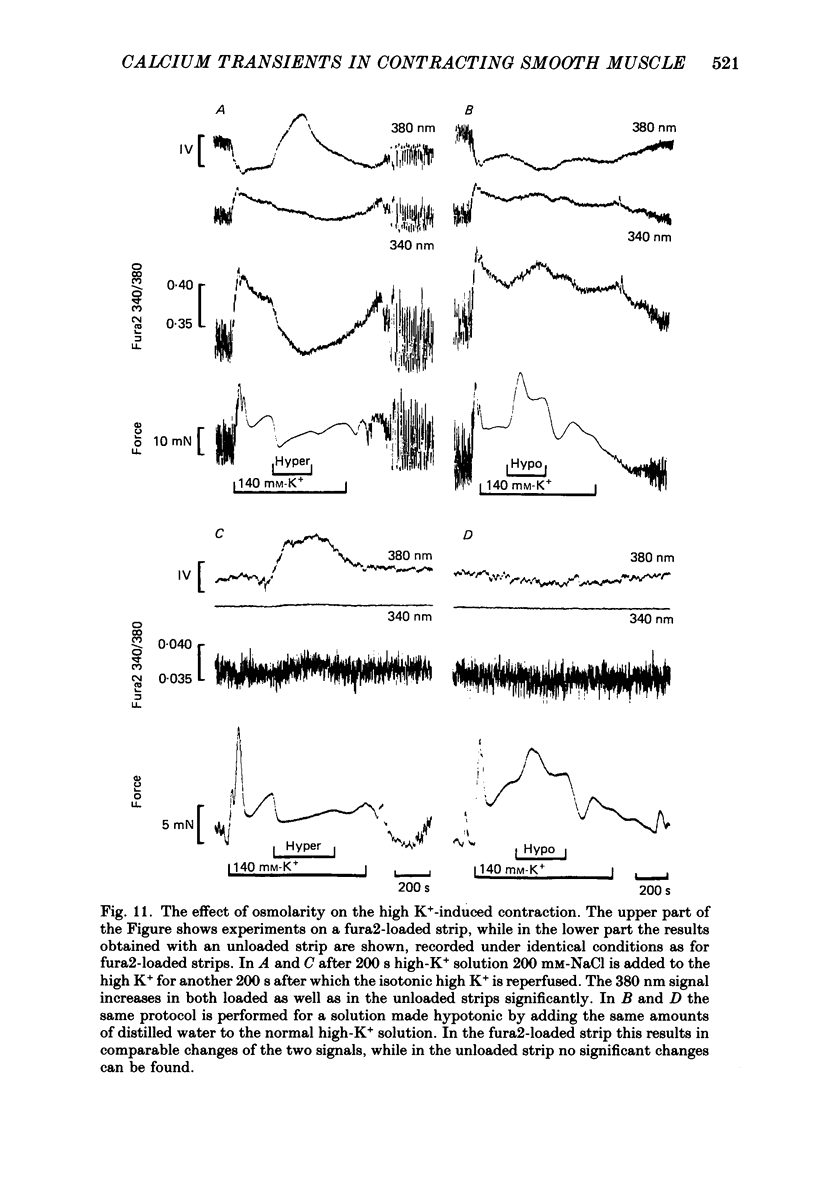
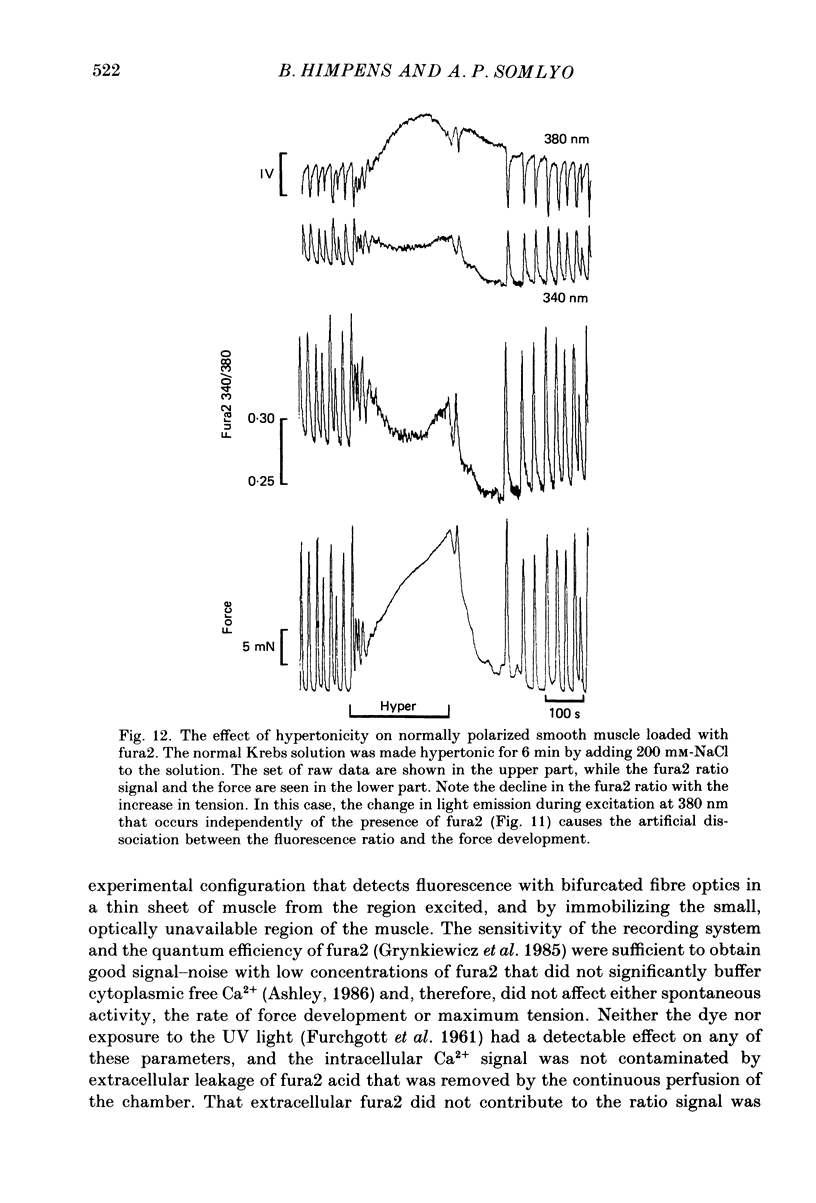








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. H. Buffer capacity of intracellular Ca2+ indicators. Biochem J. 1986 Nov 15;240(1):310–311. doi: 10.1042/bj2400310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., PROSSER C. L. Conduction in smooth muscles: comparative electrical properties. Am J Physiol. 1960 Sep;199:553–559. doi: 10.1152/ajplegacy.1960.199.3.553. [DOI] [PubMed] [Google Scholar]
- Bitar K. N., Bradford P., Putney J. W., Jr, Makhlouf G. M. Cytosolic calcium during contraction of isolated mammalian gastric muscle cells. Science. 1986 May 30;232(4754):1143–1145. doi: 10.1126/science.3704641. [DOI] [PubMed] [Google Scholar]
- Bohr D. F. Vascular Smooth Muscle: Dual Effect of Calcium. Science. 1963 Feb 15;139(3555):597–599. doi: 10.1126/science.139.3555.597. [DOI] [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of temperature on the membrane conductance of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):621–635. doi: 10.1113/jphysiol.1969.sp008712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S. Effects of phosphorylation, calcium ion, and tropomyosin on actin-activated adenosine 5'-triphosphatase activity of mammalian smooth muscle myosin. Biochemistry. 1981 Feb 17;20(4):702–707. doi: 10.1021/bi00507a005. [DOI] [PubMed] [Google Scholar]
- Chance B., Legallais V., Sorge J., Graham N. A versatile time-sharing multichannel spectrophotometer, reflectometer, and fluorometer. Anal Biochem. 1975 Jun;66(2):498–514. doi: 10.1016/0003-2697(75)90617-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Tejada M. Phorbol ester-induced contraction in chemically skinned vascular smooth muscle. Am J Physiol. 1986 Sep;251(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1986.251.3.C356. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Kreulen D., Prosser C. L., Weigel R. Interaction between longitudinal and circular muscle in intestine of cat. J Physiol. 1977 Dec;273(3):665–689. doi: 10.1113/jphysiol.1977.sp012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J Physiol. 1985 Dec;369:269–282. doi: 10.1113/jphysiol.1985.sp015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon P. F., Aksoy M. O., Driska S. P., Murphy R. A. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981 Jan 30;211(4481):495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel S., Schütz H., Hornitschek A. Untersuchungen zur Analytik von Nifedipin unter besonderer Berücksichtigung der bei Lichtexposition entstehenden Umwandlungsprodukte. Arzneimittelforschung. 1978;28(12):2188–2193. [PubMed] [Google Scholar]
- Eisenberg B. R., Mathias R. T., Gilai A. Intracellular localization of markers within injected or cut frog muscle fibers. Am J Physiol. 1979 Jul;237(1):C50–C55. doi: 10.1152/ajpcell.1979.237.1.C50. [DOI] [PubMed] [Google Scholar]
- FILO R. S., BOHR D. F., RUEGG J. C. GLYCERINATED SKELETAL AND SMOOTH MUSCLE: CALCIUM AND MAGNESIUM DEPENDENCE. Science. 1965 Mar 26;147(3665):1581–1583. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R. F., EHRREICH S. J., GREENBLATT E. The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol. 1961 Jan;44:499–519. doi: 10.1085/jgp.44.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Shlevin H. H., Granger W. C., Jr, Taylor S. R. Aequorin luminescence during activation of single isolated smooth muscle cells. Nature. 1979 Aug 9;280(5722):506–508. doi: 10.1038/280506a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Himpens B., Casteels R. Measurement by Quin2 of changes of the intracellular calcium concentration in strips of the rabbit ear artery and of the guinea-pig ileum. Pflugers Arch. 1987 Jan;408(1):32–37. doi: 10.1007/BF00581837. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Kerrick W. G., Cassidy P. S. Chicken gizzard: relation between calcium-activated phosphorylation and contraction. Science. 1979 May 4;204(4392):503–506. doi: 10.1126/science.432654. [DOI] [PubMed] [Google Scholar]
- Hurwitz L., Joiner P. D. Excitation-contraction coupling in smooth muscle. Fed Proc. 1969 Sep-Oct;28(5):1629–1633. [PubMed] [Google Scholar]
- Karlsson J. O., Axelsson K. L., Elwing H., Andersson R. G. Action spectra of photoactivated cyclic GMP metabolism and relaxation in bovine mesenteric artery. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(3):155–166. [PubMed] [Google Scholar]
- Kitazawa T. Effect of extracellular calcium on contractile activation in guinea-pig ventricular muscle. J Physiol. 1984 Oct;355:635–659. doi: 10.1113/jphysiol.1984.sp015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kanaide H., Nakamura M. K+-depolarization induces a direct release of Ca2+ from intracellular storage sites in cultured vascular smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1985 Jun 28;129(3):877–884. doi: 10.1016/0006-291x(85)91973-4. [DOI] [PubMed] [Google Scholar]
- Kowarski D., Shuman H., Somlyo A. P., Somlyo A. V. Calcium release by noradrenaline from central sarcoplasmic reticulum in rabbit main pulmonary artery smooth muscle. J Physiol. 1985 Sep;366:153–175. doi: 10.1113/jphysiol.1985.sp015790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A. Measuring cytosolic free calcium concentration in endothelial cells with indo-1: the pitfall of using the ratio of two fluorescence intensities recorded at different wavelengths. Cell Calcium. 1986 Aug;7(4):233–248. doi: 10.1016/0143-4160(86)90003-5. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. The thin filaments of smooth muscles. J Muscle Res Cell Motil. 1985 Dec;6(6):669–708. doi: 10.1007/BF00712237. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. A., Aksoy M. O., Dillon P. F., Gerthoffer W. T., Kamm K. E. The role of myosin light chain phosphorylation in regulation of the cross-bridge cycle. Fed Proc. 1983 Jan;42(1):51–56. [PubMed] [Google Scholar]
- Neering I. R., Morgan K. G. Use of aequorin to study excitation--contraction coupling in mammalian smooth muscle. Nature. 1980 Dec 11;288(5791):585–587. doi: 10.1038/288585a0. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Pritchard K., Ashley C. C. Na+/Ca2+ exchange in isolated smooth muscle cells demonstrated by the fluorescent calcium indicator fura-2. FEBS Lett. 1986 Jan 20;195(1-2):23–27. doi: 10.1016/0014-5793(86)80122-3. [DOI] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res. 1986 Jun;58(6):803–815. doi: 10.1161/01.res.58.6.803. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Pozzan T. Using quin2 in cell suspensions. Cell Calcium. 1985 Apr;6(1-2):133–144. doi: 10.1016/0143-4160(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Stumpf H. Activation of the myofibrillar ATPase activity by extension of glycerolextracted insect fibrillar muscle. Pflugers Arch. 1969;305(1):34–46. doi: 10.1007/BF00586394. [DOI] [PubMed] [Google Scholar]
- Schackmann R. W., Chock P. B. Alteration of intracellular [Ca2+] in sea urchin sperm by the egg peptide speract. Evidence that increased intracellular Ca2+ is coupled to Na+ entry and increased intracellular pH. J Biol Chem. 1986 Jul 5;261(19):8719–8728. [PubMed] [Google Scholar]
- Sobieszek A. Ca-linked phosphorylation of a light chain of vertebrate smooth-muscle myosin. Eur J Biochem. 1977 Mar 1;73(2):477–483. doi: 10.1111/j.1432-1033.1977.tb11340.x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P. Excitation-contraction coupling and the ultrastructure of smooth muscle. Circ Res. 1985 Oct;57(4):497–507. doi: 10.1161/01.res.57.4.497. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinsland O. S., Furchgott R. F., Kirpekar S. M. Biphasic vasoconstriction of the rabbit ear artery. Circ Res. 1973 Jan;32(1):49–58. doi: 10.1161/01.res.32.1.49. [DOI] [PubMed] [Google Scholar]
- Sumimoto K., Kuriyama H. Mobilization of free Ca2+ measured during contraction-relaxation cycles in smooth muscle cells of the porcine coronary artery using quin2. Pflugers Arch. 1986 Feb;406(2):173–180. doi: 10.1007/BF00586679. [DOI] [PubMed] [Google Scholar]
- Syson A. J., Huddart H. Contracture tension in rat vas deferens and ileal smooth muscle and its modification by external calcium and the tonicity of the medium. Comp Biochem Physiol A Comp Physiol. 1973 Jun 1;45(2):345–362. doi: 10.1016/0300-9629(73)90440-4. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- URAKAWA N., HOLLAND W. C. CA45 UPTAKE AND TISSUE CALCIUM IN K-INDUCED PHASIC AND TONIC CONTRACTION IN TAENIA COLI. Am J Physiol. 1964 Oct;207:873–876. doi: 10.1152/ajplegacy.1964.207.4.873. [DOI] [PubMed] [Google Scholar]
- Wagner J., Rüegg J. C. Skinned smooth muscle: calcium-calmodulin activation independent of myosin phosphorylation. Pflugers Arch. 1986 Nov;407(5):569–571. doi: 10.1007/BF00657520. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Somlyo A. V., Goldman Y. E., Somlyo A. P., Trentham D. R. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987 May 21;327(6119):249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Calcium transients and resting levels in isolated smooth muscle cells as monitored with quin 2. Am J Physiol. 1986 May;250(5 Pt 1):C779–C791. doi: 10.1152/ajpcell.1986.250.5.C779. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H. Recording of intracellular Ca2+ from smooth muscle cells by sub-micron tip, double-barrelled CA2+-selective microelectrodes. Cell Calcium. 1986 Aug;7(4):203–219. doi: 10.1016/0143-4160(86)90001-1. [DOI] [PubMed] [Google Scholar]



