Abstract
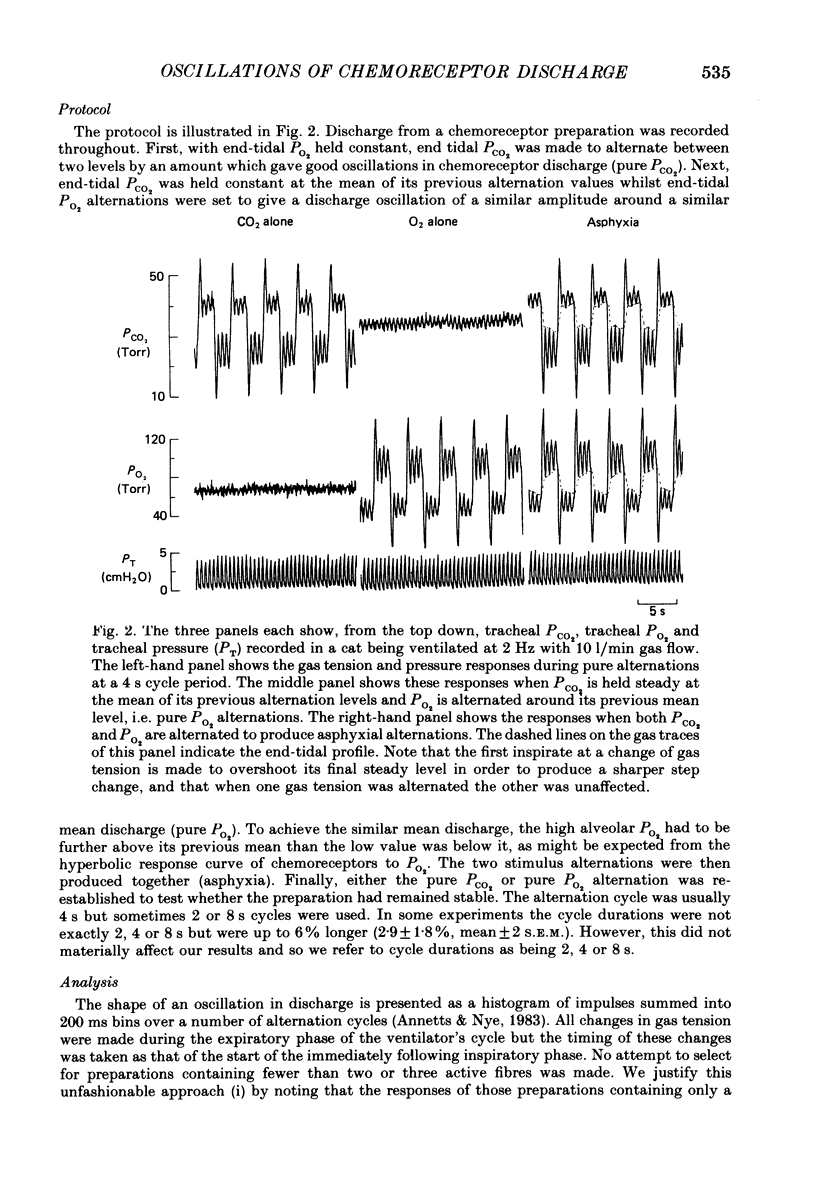
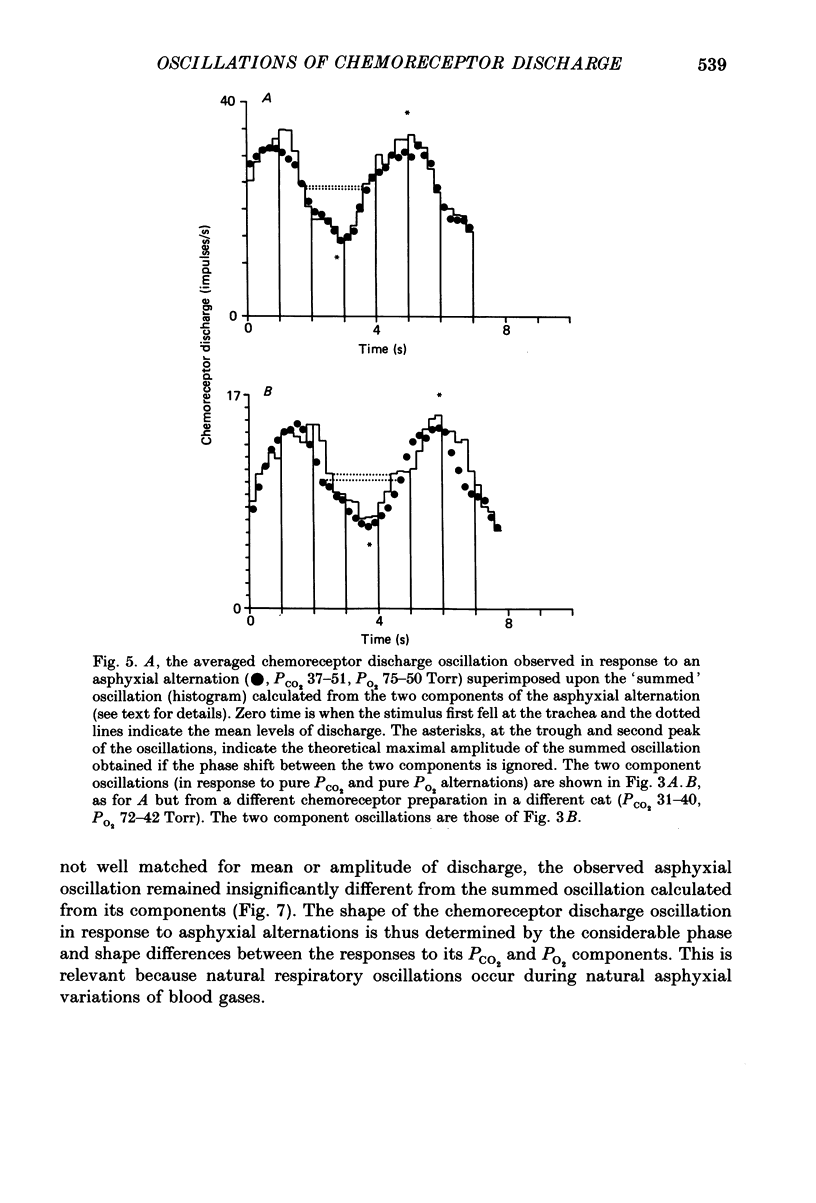
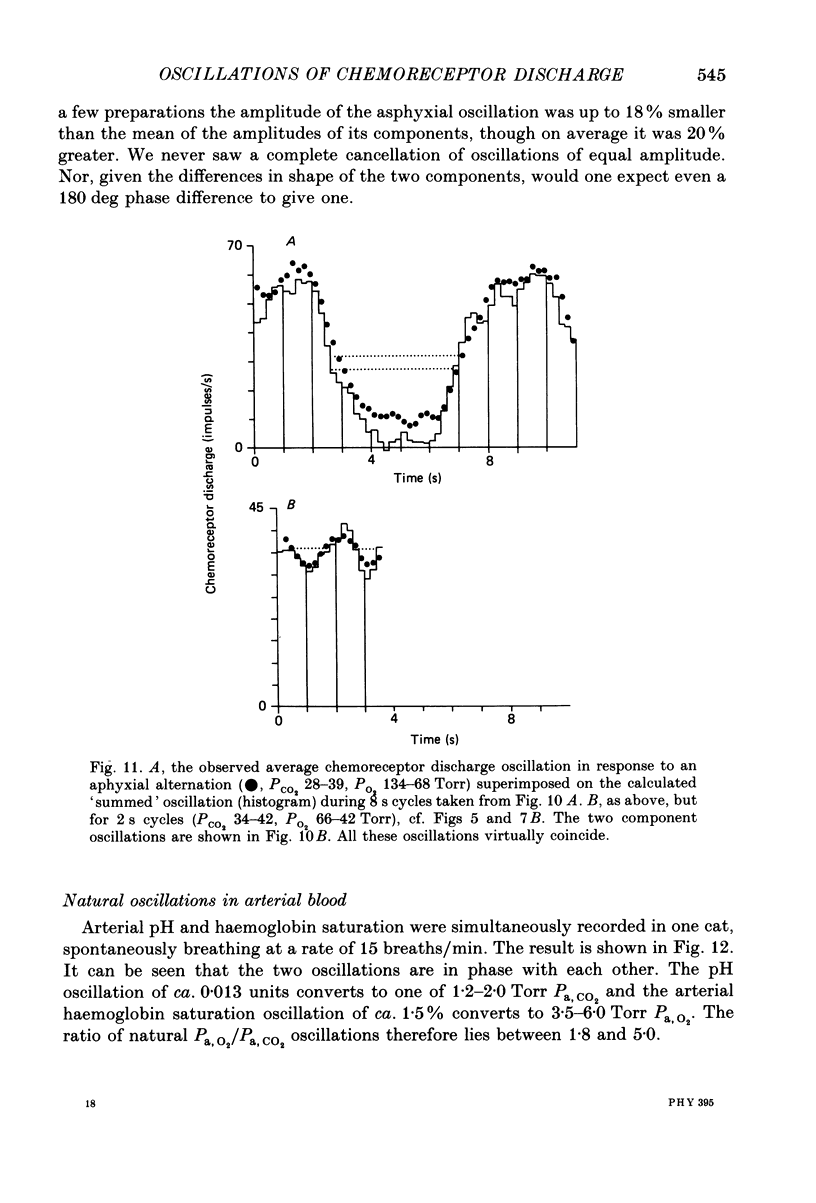
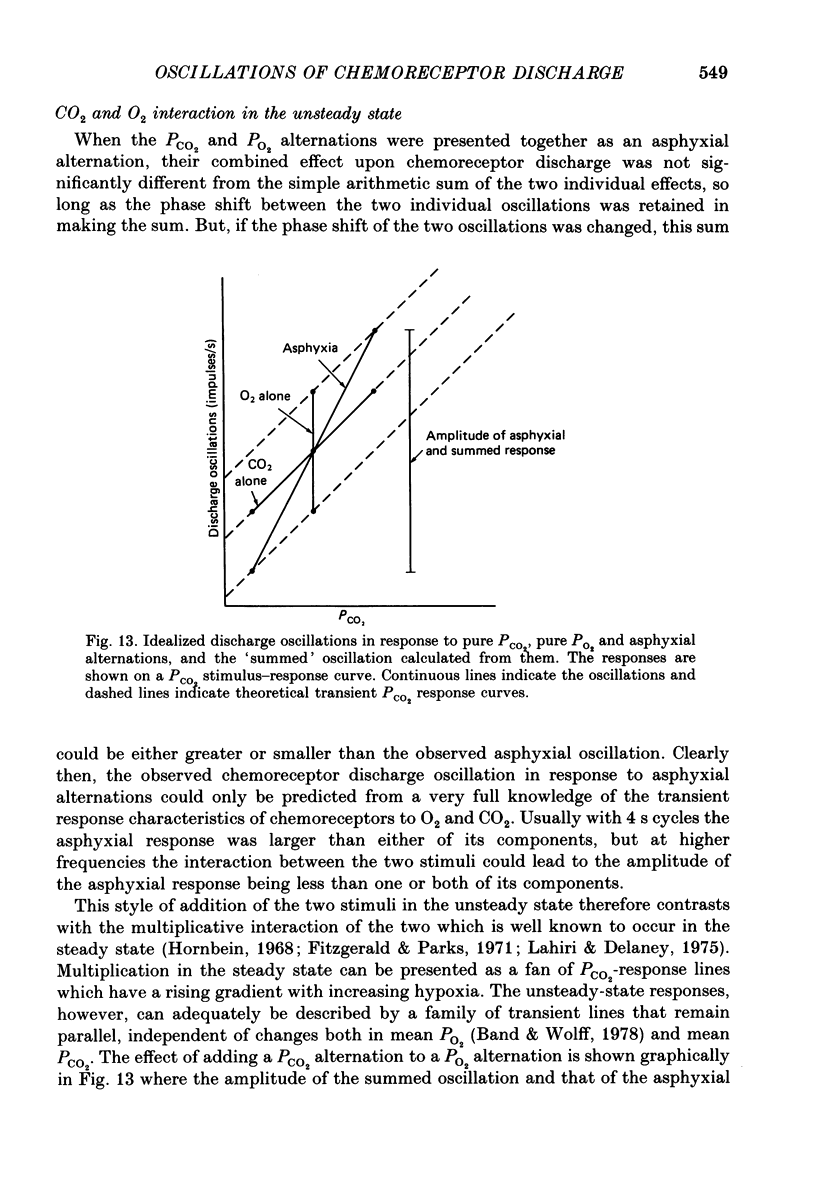
1. A high-frequency high-flow ventilator has been developed which will produce abrupt changes in alveolar gas tensions. We have used it to study the individual contributions of PCO2 and PO2 in producing the oscillations which occur in the discharge of carotid chemoreceptors in the cat with respiration, by producing repeated end-tidal alternations (i) of PCO2 in constant hypoxia, (ii) of PO2 in constant normocapnia and (iii) of both PO2 and PCO2, i.e. of asphyxia. 2. The chemoreceptor response to alternations of PCO2 was always brisker than that to alternations of PO2 at 2, 4 or 8 s cycle durations. 3. An increase in the frequency of the alveolar alternation shortened the difference between the response times to PCO2 and PO2 but it increased the phase difference between the stimulus and the response waveforms. 4. With 4 s cycles, in normocapnic hypoxia, PCO2 was 2.9 times more effective (impulses s-1 Torr-1) than PO2 in producing oscillations in discharge. 5. The oscillations in discharge to simultaneous alternations of PO2 and PCO2 were not significantly different from the sum of individual oscillations to alternations of PCO2 and of PO2 alone. This was true with respect to timing and to amplitude of the oscillation. 6. Usually the amplitude of the chemoreceptor discharge oscillation in response to an asphyxial alternation was greater than the amplitude of the oscillation to either its PCO2 or its PO2 component alone. However, at the highest frequencies used, the phase relation between the PCO2 and PO2 components of the response could lead to the summed asphyxial response being less than its individual components. 7. The amplitudes and shapes of the oscillations in response to 4 s PCO2 alternations were not affected by changing either the steady-background PO2 or PCO2, but the amplitudes of the oscillations to pure PO2 alternations were enhanced by hypoxia and by hypercapnia. The importance of PO2 and PCO2 in giving rise to the natural respiratory oscillations in chemoreceptor discharge depends on the mean levels of the two gases. In normocapnic hypoxia (PO2 ca. 50 Torr) they are equally important but when PO2 is raised it becomes less important.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken R. S., Clark-Kennedy A. E. On the fluctuation in the composition of the alveolar air during the respiratory cycle in muscular exercise. J Physiol. 1928 Aug 14;65(4):389–411. doi: 10.1113/jphysiol.1928.sp002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band D. M., Wolff C. B. Respiratory oscillations in discharge frequency of chemoreceptor afferents in sinus nerve and anaesthetized cats at normal and low arterial oxygen tensions. J Physiol. 1978 Sep;282:1–6. doi: 10.1113/jphysiol.1978.sp012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A. M., McCloskey D. I., Torrance R. W. The responses of carotid body chemoreceptors in the cat to sudden changes of hypercapnic and hypoxic stimuli. Respir Physiol. 1971 Oct;13(1):36–49. doi: 10.1016/0034-5687(71)90063-6. [DOI] [PubMed] [Google Scholar]
- Brown A. M. Receptors under pressure. An update on baroreceptors. Circ Res. 1980 Jan;46(1):1–10. doi: 10.1161/01.res.46.1.1. [DOI] [PubMed] [Google Scholar]
- Cross B. A., Leaver K. D., Semple S. J., Stidwill R. P. The effect of small changes in arterial carbon dioxide tension on carotid chemoreceptor activity in the cat. J Physiol. 1986 Nov;380:415–427. doi: 10.1113/jphysiol.1986.sp016294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS A. B. Alveolar CO2 and O2 during breath holding, expiration, and inspiration. J Appl Physiol. 1952 Jul;5(1):1–12. doi: 10.1152/jappl.1952.5.1.1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. S., Leitner L. M., Liaubet M. J. Carotid chemoreceptor response to intermittent or sustained stimulation in the cat. Respir Physiol. 1969 Apr;6(3):395–402. doi: 10.1016/0034-5687(69)90037-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. S., Parks D. C. Effect of hypoxia on carotid chemoreceptor response to carbon dioxide in cats. Respir Physiol. 1971 Jun;12(2):218–229. doi: 10.1016/0034-5687(71)90054-5. [DOI] [PubMed] [Google Scholar]
- Gehrich J. L., Moore G. P. Statistical analysis of cyclic variations in carotid body chemoreceptor activity. J Appl Physiol. 1973 Nov;35(5):642–648. doi: 10.1152/jappl.1973.35.5.642. [DOI] [PubMed] [Google Scholar]
- Goodman N. W. Efferent control of arterial chemoreceptors mediated by glossopharyngeal fibres and artifacts introduced by stimulation techniques. J Physiol. 1973 Apr;230(2):295–311. doi: 10.1113/jphysiol.1973.sp010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman N. W., Nail B. S., Torrance R. W. Oscillations in the discharge of single carotid chemorecptor fibers of the cat. Respir Physiol. 1974 Jun;20(3):251–269. doi: 10.1016/0034-5687(74)90023-1. [DOI] [PubMed] [Google Scholar]
- Goodman N. W. Some observations on the homogeneity of response of single chemoreceptor fibres. Respir Physiol. 1974 Jun;20(3):271–281. doi: 10.1016/0034-5687(74)90024-3. [DOI] [PubMed] [Google Scholar]
- Haldane J. S., Priestley J. G. The regulation of the lung-ventilation. J Physiol. 1905 May 9;32(3-4):225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A., Lindhard J. On the average composition of the alveolar air and its variations during the respiratory cycle. J Physiol. 1914 Feb 27;47(6):431–445. doi: 10.1113/jphysiol.1914.sp001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S., DeLaney R. G. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975 Sep;24(3):249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Mulligan E., Mokashi A. Adaptive response of carotid body chemoreceptors to CO2. Brain Res. 1982 Feb 18;234(1):137–147. doi: 10.1016/0006-8993(82)90478-4. [DOI] [PubMed] [Google Scholar]
- Ponte J., Purves M. J. Frequency response of carotid body chemoreceptors in the cat to changes of PaCO2, PaO2, and pHa. J Appl Physiol. 1974 Nov;37(5):635–647. doi: 10.1152/jappl.1974.37.5.635. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Swanson G. D., Micco A. J., Schubert W. P. A fast gas-mixing system for breath-to-breath respiratory control studies. J Appl Physiol Respir Environ Exerc Physiol. 1982 May;52(5):1358–1362. doi: 10.1152/jappl.1982.52.5.1358. [DOI] [PubMed] [Google Scholar]
- Scheid P., Piiper J. Blood/gas equilibrium of carbon dioxide in lungs. A critical review. Respir Physiol. 1980 Jan;39(1):1–31. doi: 10.1016/0034-5687(80)90011-0. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO W. S. Transmission of information by the arterial blood stream with particular reference to carbon dioxide. Biophys J. 1962 Mar;2:143–159. doi: 10.1016/s0006-3495(62)86846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota H., Kreuzer F. Alveolar to arterial transmission of oxygen fluctuations due to respiration in anesthetized dogs. Pflugers Arch. 1973 Jun 4;340(4):291–306. doi: 10.1007/BF00592308. [DOI] [PubMed] [Google Scholar]



