Abstract
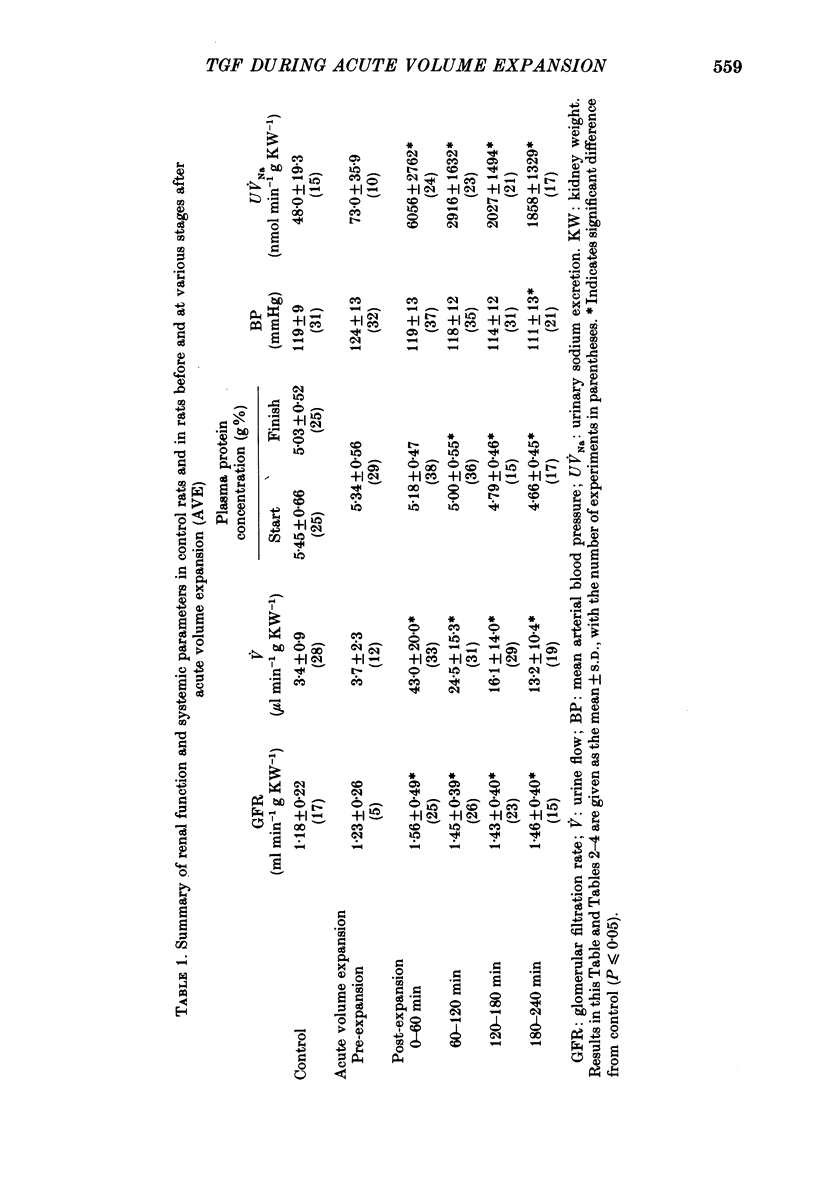
1. Volume expansion is currently believed to change the intrinsic properties of the juxtaglomerular apparatus such that the sensitivity of the tubuloglomerular feedback (TGF) mechanism is reduced, thus allowing glomerular filtration rate, and hence salt and water excretion, to rise. Recent studies conflict with this view and indeed the older literature reveals that the rise in glomerular filtration rate (GFR) under these conditions is far more modest than would be expected if TGF control were eliminated. 2. To investigate this problem, TGF control of filtration rate was examined by measuring single-nephron glomerular filtration rate (SNGFR) during loop of Henle perfusion at varying rates in rats under control conditions, after acute, moderate (4% of body weight), iso-oncotic volume expansion and in rats treated with antibodies to atrial natriuretic peptide (ANP) prior to the acute volume expansion. 3. With TGF control of filtration interrupted by filtrate collection from the proximal tubule, SNGFR in the expanded rats was massively increased compared with controls, although SNGFR measured in the distal tubule, and hence with TGF control intact, was only modestly increased, as was whole-kidney filtration rate. Loop perfusion at increasing rates up to 30 nl min-1 progressively decreased SNGFR in controls, and in the expanded rats the range over which control was exerted extended up to 60-80 nl min-1. For changes in loop flow around the spontaneous operating point, the sensitivity of the TGF mechanism, defined as delta SNGFR/delta loop flow, was similar in both groups. Treatment of rats with ANP antibodies prior to volume expansion substantially blunted the changes in renal salt and water excretion and the increase in SNGFR seen in the absence of loop perfusion. 4. These results are not consistent with a diminution of TGF function after volume expansion, rather with an enhancement. The latter is best accounted for by vasodilation of preglomerular resistance vessels on volume expansion, a result predicted by calculations from a model based on the serial arrangement of preglomerular and TGF-controlled vascular resistance elements and the established pharmacological actions of ANP.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreucci V. E., Dal Canton A., Corradi A., Stanziale R., Migone L. Role of the efferent arteriole in glomerular hemodynamics of superficial nephrons. Kidney Int. 1976 Jun;9(6):475–480. doi: 10.1038/ki.1976.61. [DOI] [PubMed] [Google Scholar]
- Arendshorst W. J., Finn W. F. Renal hemodynamics in the rat before and during inhibition of angiotensin II. Am J Physiol. 1977 Oct;233(4):F290–F297. doi: 10.1152/ajprenal.1977.233.4.F290. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S., Wada T. Effect of peritubular capillary perfusion rate on proximal sodium reabsorption. Kidney Int. 1972 Jun;1(6):397–405. doi: 10.1038/ki.1972.52. [DOI] [PubMed] [Google Scholar]
- Baylis C., Brenner B. M. The physiologic determinants of glomerular ultrafiltration. Rev Physiol Biochem Pharmacol. 1978;80:1–46. doi: 10.1007/3540084665_1. [DOI] [PubMed] [Google Scholar]
- Baylis C., Ichikawa I., Willis W. T., Wilson C. B., Brenner B. M. Dynamics of glomerular ultrafiltration. IX. Effects of plasma protein concentration. Am J Physiol. 1977 Jan;232(1):F58–F71. doi: 10.1152/ajprenal.1977.232.1.F58. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Rector F. C., Jr, Seldin D. W. Effect of hyperoncotic albumin expansion upon glomerular ultrafiltration in the rat. Kidney Int. 1974 Oct;6(4):209–221. doi: 10.1038/ki.1974.102. [DOI] [PubMed] [Google Scholar]
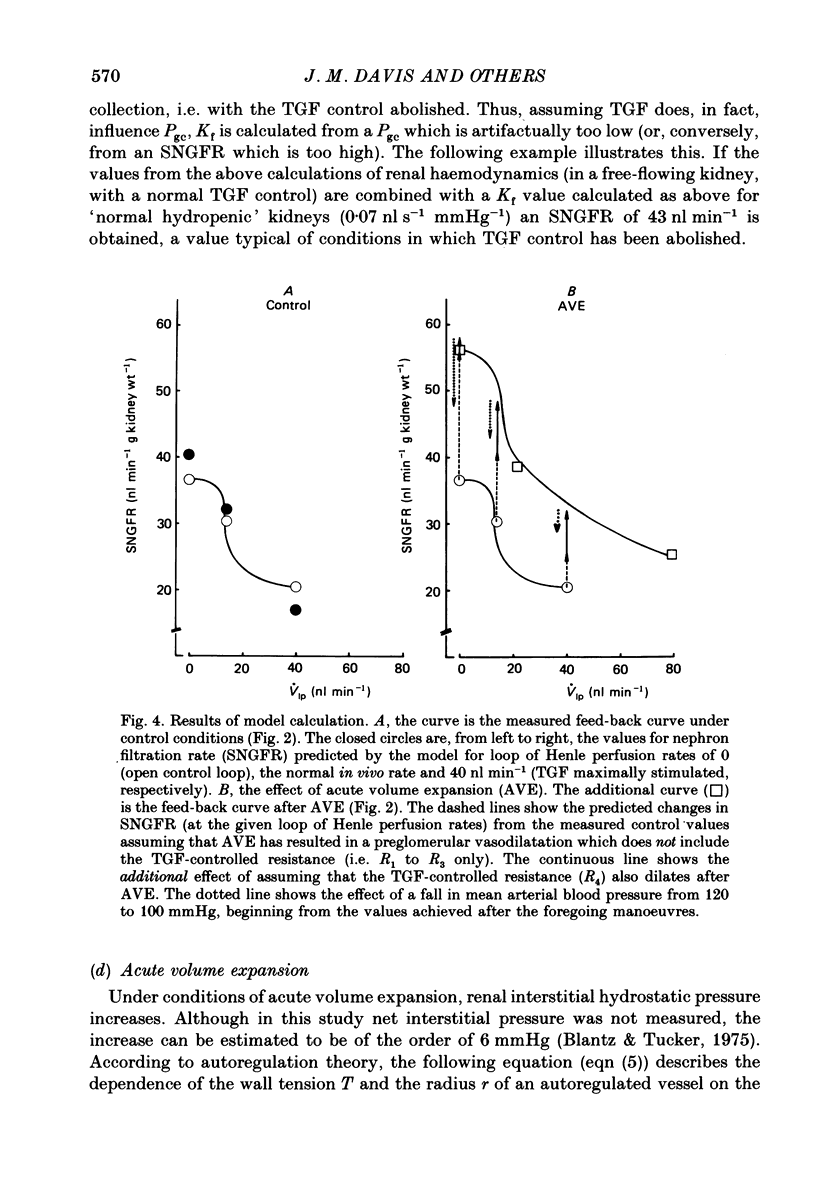
- Blantz R. C., Tucker B. J. Determinants of peritubular capillary fluid uptake in hydropenia and saline and plasma expansion. Am J Physiol. 1975 Jun;228(6):1927–1935. doi: 10.1152/ajplegacy.1975.228.6.1927. [DOI] [PubMed] [Google Scholar]
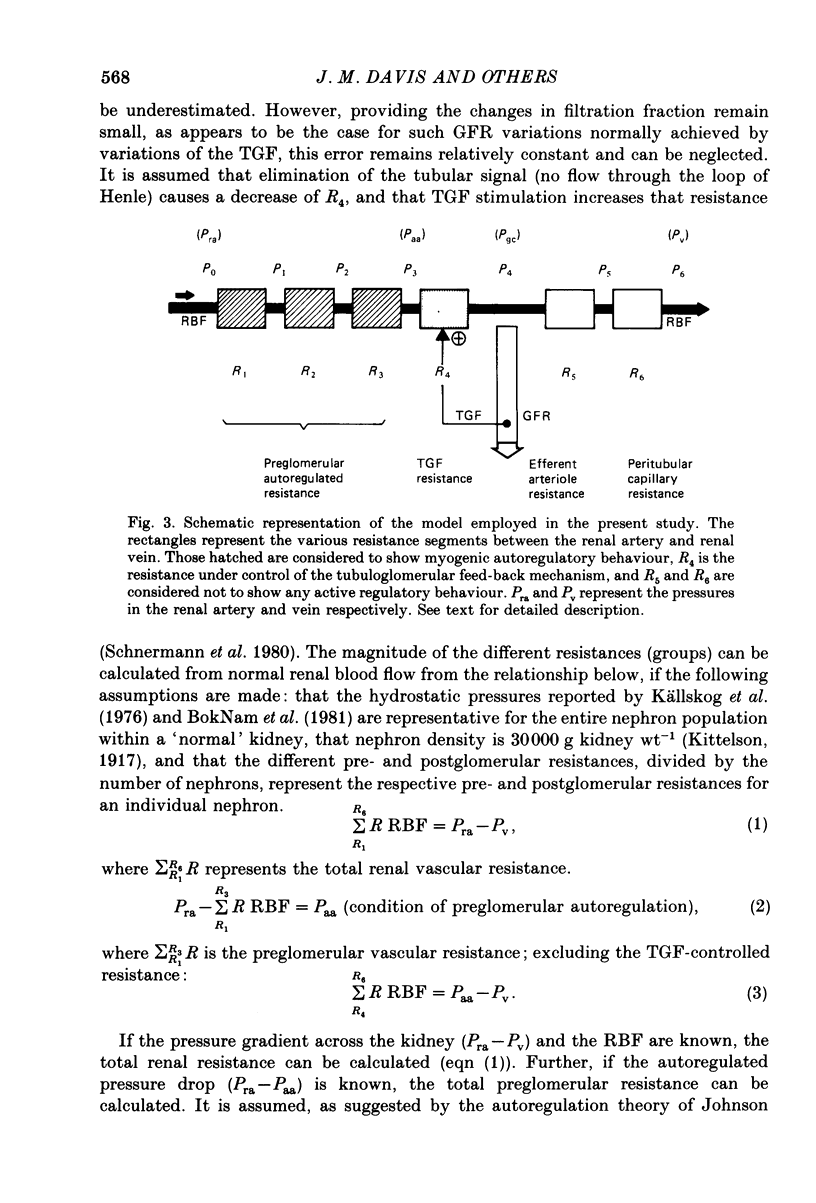
- Boknam L., Ericson A. C., Aberg B., Ulfendahl H. R. Flow resistance of the interlobular artery in the rat kidney. Acta Physiol Scand. 1981 Feb;111(2):159–163. doi: 10.1111/j.1748-1716.1981.tb06719.x. [DOI] [PubMed] [Google Scholar]
- Briggs J. P., Steipe B., Schubert G., Schnermann J. Micropuncture studies of the renal effects of atrial natriuretic substance. Pflugers Arch. 1982 Dec;395(4):271–276. doi: 10.1007/BF00580789. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Duchin K. L., Peterson L. N., Burke T. J. Effect of furosemide on renal autoregulation. Kidney Int. 1977 Dec;12(6):379–386. doi: 10.1038/ki.1977.128. [DOI] [PubMed] [Google Scholar]
- Gertz K. H., Mangos J. A., Braun G., Pagel H. D. Pressure in the glomerular capillaries of the rat kidney and its relation to arterial blood pressure. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;288(4):369–374. doi: 10.1007/BF00362581. [DOI] [PubMed] [Google Scholar]
- Hirth C., Stasch J. P., John A., Kazda S., Morich F., Neuser D., Wohlfeil S. The renal response to acute hypervolemia is caused by atrial natriuretic peptides. J Cardiovasc Pharmacol. 1986 Mar-Apr;8(2):268–275. doi: 10.1097/00005344-198603000-00008. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Cogan M. G. Atrial natriuretic factor inhibits maximal tubuloglomerular feedback response. Am J Physiol. 1987 May;252(5 Pt 2):F825–F828. doi: 10.1152/ajprenal.1987.252.5.F825. [DOI] [PubMed] [Google Scholar]
- Häberle D. A., Davis J. M. Chronic salt loading: effects on plasma volume and regulation of glomerular filtration rate in Wistar rats. Klin Wochenschr. 1982 Oct 1;60(19):1245–1248. doi: 10.1007/BF01716731. [DOI] [PubMed] [Google Scholar]
- Häberle D. A., Davis J. M. Resetting of tubuloglomerular feedback: evidence for a humoral factor in tubular fluid. Am J Physiol. 1984 Apr;246(4 Pt 2):F495–F500. doi: 10.1152/ajprenal.1984.246.4.F495. [DOI] [PubMed] [Google Scholar]
- John A., Stasch J. P., Neuser D., Hirth C., Morich F. J. The use of a monoclonal antibody to measure plasma atriopeptins in rat. Life Sci. 1986 Jun 2;38(22):1991–1997. doi: 10.1016/0024-3205(86)90146-3. [DOI] [PubMed] [Google Scholar]
- Källskog O., Lindbrom L. O., Ulfendahl H. R., Wolgast M. Hydrostatic pressures within the vascular structures of the rat kidney. Pflugers Arch. 1976 Jun 22;363(3):205–210. doi: 10.1007/BF00594602. [DOI] [PubMed] [Google Scholar]
- Lang R. E., Thölken H., Ganten D., Luft F. C., Ruskoaho H., Unger T. Atrial natriuretic factor--a circulating hormone stimulated by volume loading. Nature. 1985 Mar 21;314(6008):264–266. doi: 10.1038/314264a0. [DOI] [PubMed] [Google Scholar]
- Luft F. C., Sterzel R. B., Lang R. E., Trabold E. M., Veelken R., Ruskoaho H., Gao Y., Ganten D., Unger T. Atrial natriuretic factor determinations and chronic sodium homeostasis. Kidney Int. 1986 May;29(5):1004–1010. doi: 10.1038/ki.1986.100. [DOI] [PubMed] [Google Scholar]
- Marin-Grez M., Fleming J. T., Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature. 1986 Dec 4;324(6096):473–476. doi: 10.1038/324473a0. [DOI] [PubMed] [Google Scholar]
- Moore L. C., Mason J. Tubuloglomerular feedback control of distal fluid delivery: effect of extracellular volume. Am J Physiol. 1986 Jun;250(6 Pt 2):F1024–F1032. doi: 10.1152/ajprenal.1986.250.6.F1024. [DOI] [PubMed] [Google Scholar]
- Moore L. C. Tubuloglomerular feedback and SNGFR autoregulation in the rat. Am J Physiol. 1984 Aug;247(2 Pt 2):F267–F276. doi: 10.1152/ajprenal.1984.247.2.F267. [DOI] [PubMed] [Google Scholar]
- Müller-Suur R., Gutsche H. U., Samwer K. F., Oelkers W., Hierholzer K. Tubuloglomerular feedback in rat kidneys of different renin contents. Pflugers Arch. 1975 Aug 29;359(1-2):33–56. doi: 10.1007/BF00581276. [DOI] [PubMed] [Google Scholar]
- Navar L. G., Bell P. D., Burke T. J. Role of a macula densa feedback mechanism as a mediator of renal autoregulation. Kidney Int Suppl. 1982 Aug;12:S157–S164. [PubMed] [Google Scholar]
- Persson A. E., Boberg U., Hahne B., Müller-Suur R., Norlén B. J., Selén G. Interstitial pressure as a modulator of tubuloglomerular feedback control. Kidney Int Suppl. 1982 Aug;12:S122–S128. [PubMed] [Google Scholar]
- Persson A. E., Gushwa L. C., Blantz R. C. Feedback pressure-flow responses in normal and angiotensin-prostaglandin-blocked rats. Am J Physiol. 1984 Dec;247(6 Pt 2):F925–F931. doi: 10.1152/ajprenal.1984.247.6.F925. [DOI] [PubMed] [Google Scholar]
- Persson A. E., Müller-Suur R., Selén G. Capillary oncotic pressure as a modifier for tubuloglomerular feedback. Am J Physiol. 1979 Feb;236(2):F97–102. doi: 10.1152/ajprenal.1979.236.2.F97. [DOI] [PubMed] [Google Scholar]
- Persson A. E., Schnermann J., Wright F. S. Modification of feedback influence on glomerular filtration rate by acute isotonic extracellular volume expansion. Pflugers Arch. 1979 Aug;381(2):99–105. doi: 10.1007/BF00582339. [DOI] [PubMed] [Google Scholar]
- Ploth D. W., Rudulph J., Thomas C., Navar L. G. Renal and tubuloglomerular feedback responses to plasma expansion in the rat. Am J Physiol. 1978 Aug;235(2):F156–F162. doi: 10.1152/ajprenal.1978.235.2.F156. [DOI] [PubMed] [Google Scholar]
- Ploth D. W., Schnermann J., Dahlheim H., Hermle M., Schmidmeier E. Autoregulation and tubuloglomerular feedback in normotensive and hypertensive rats. Kidney Int. 1977 Oct;12(4):253–267. doi: 10.1038/ki.1977.110. [DOI] [PubMed] [Google Scholar]
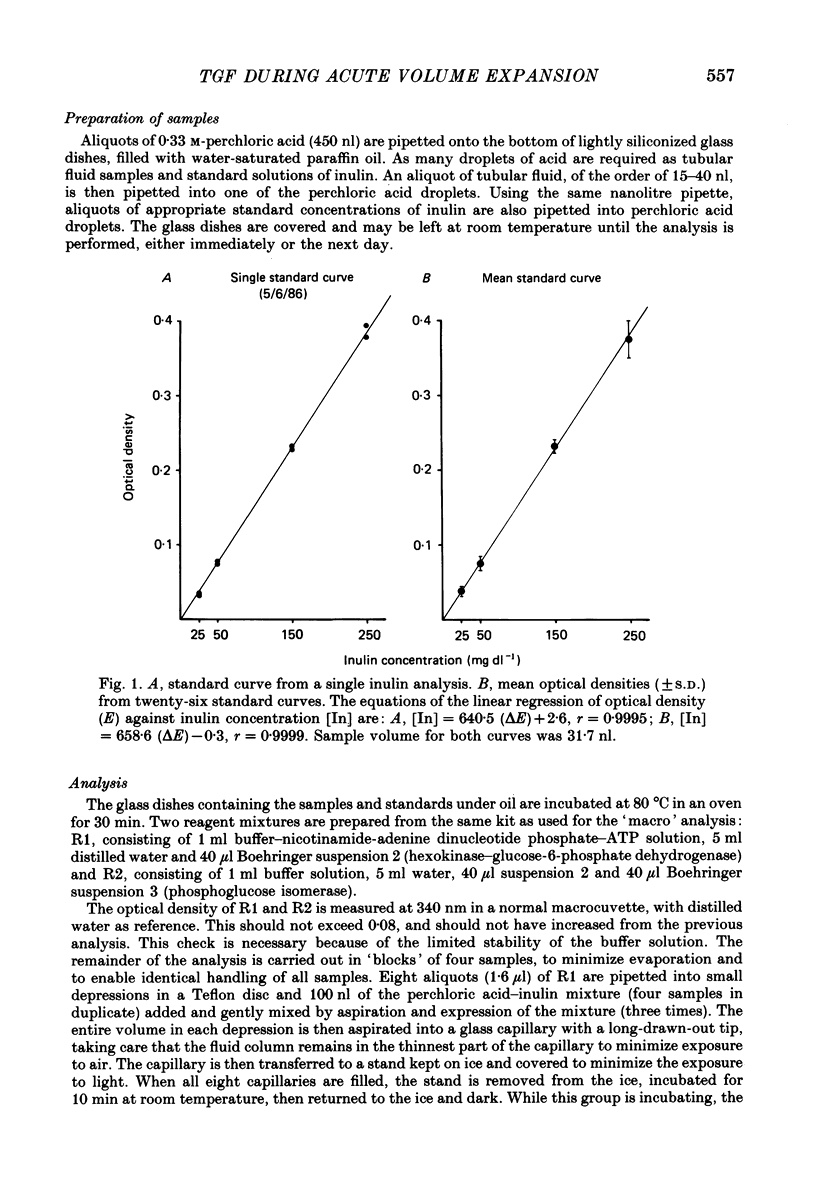
- RENSCHLER H. E. [Use of enzymatic methods in the determination of inulin]. Klin Wochenschr. 1963 Jun 15;41:615–618. doi: 10.1007/BF01487418. [DOI] [PubMed] [Google Scholar]
- Schneider W., Greger R. An improved microcuvette for photometric measurements. Pflugers Arch. 1969;311(3):268–271. doi: 10.1007/BF00590531. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Hermle M., Schmidmeier E., Dahlheim H. Impaired potency for feedback regulation of glomerular filtration rate in DOCA escaped rats. Pflugers Arch. 1975 Aug 12;358(4):325–338. doi: 10.1007/BF00580530. [DOI] [PubMed] [Google Scholar]
- Thurau K. Modification of angiotensin-mediated tubulo-glomerular feedback by extracellular volume. Kidney Int Suppl. 1975 Sep;:S202–S207. [PubMed] [Google Scholar]
- Zwiebel R., Höhmann B., Frohnert P., Baumann K. Fluorometrisch-enzymatische Mikro- und Ultramikrobestimmung von Inulin und Glucose. Pflugers Arch. 1969;307(2):127–132. [PubMed] [Google Scholar]




