Abstract
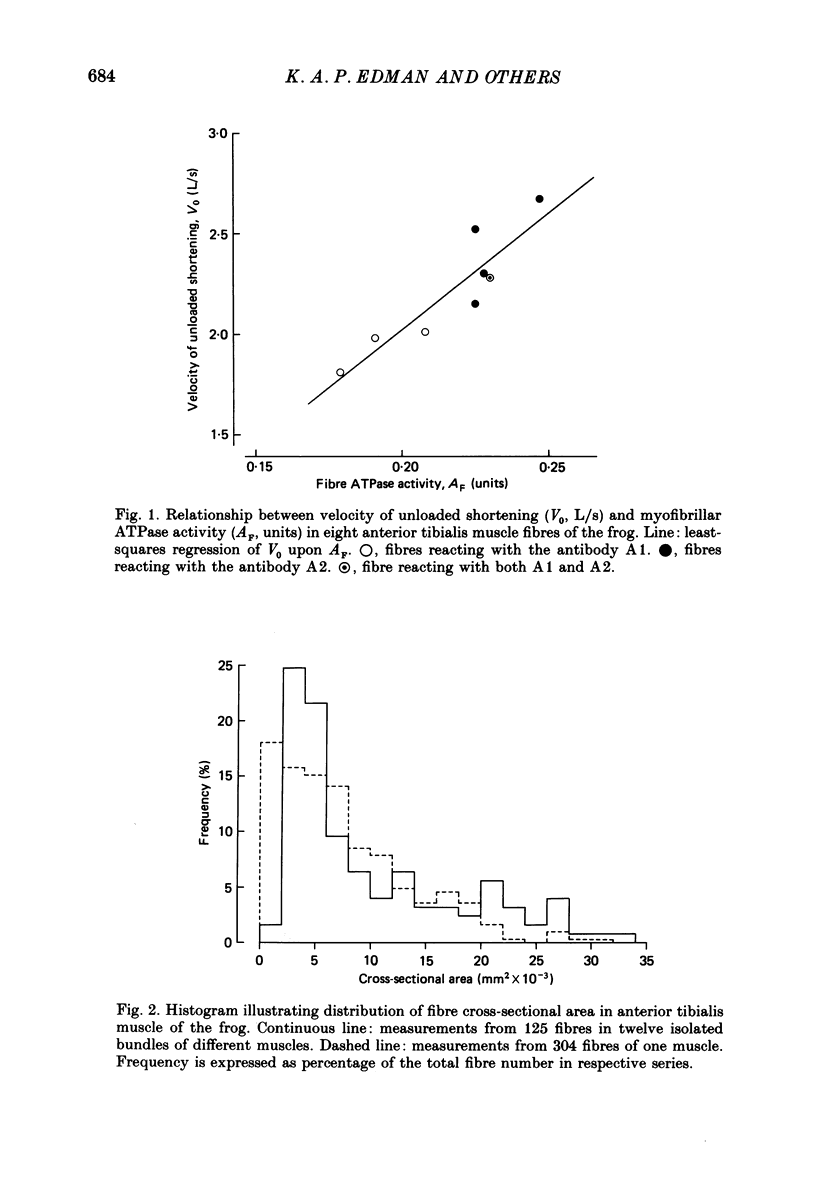
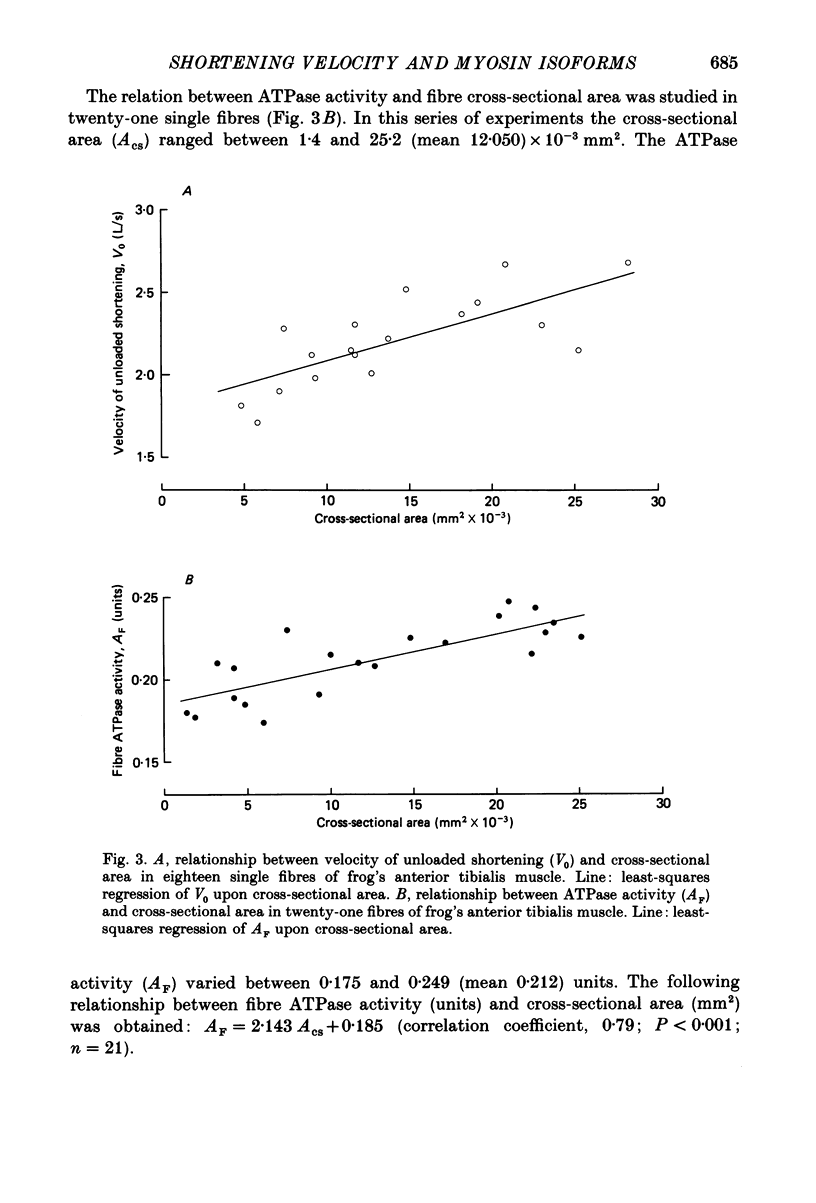
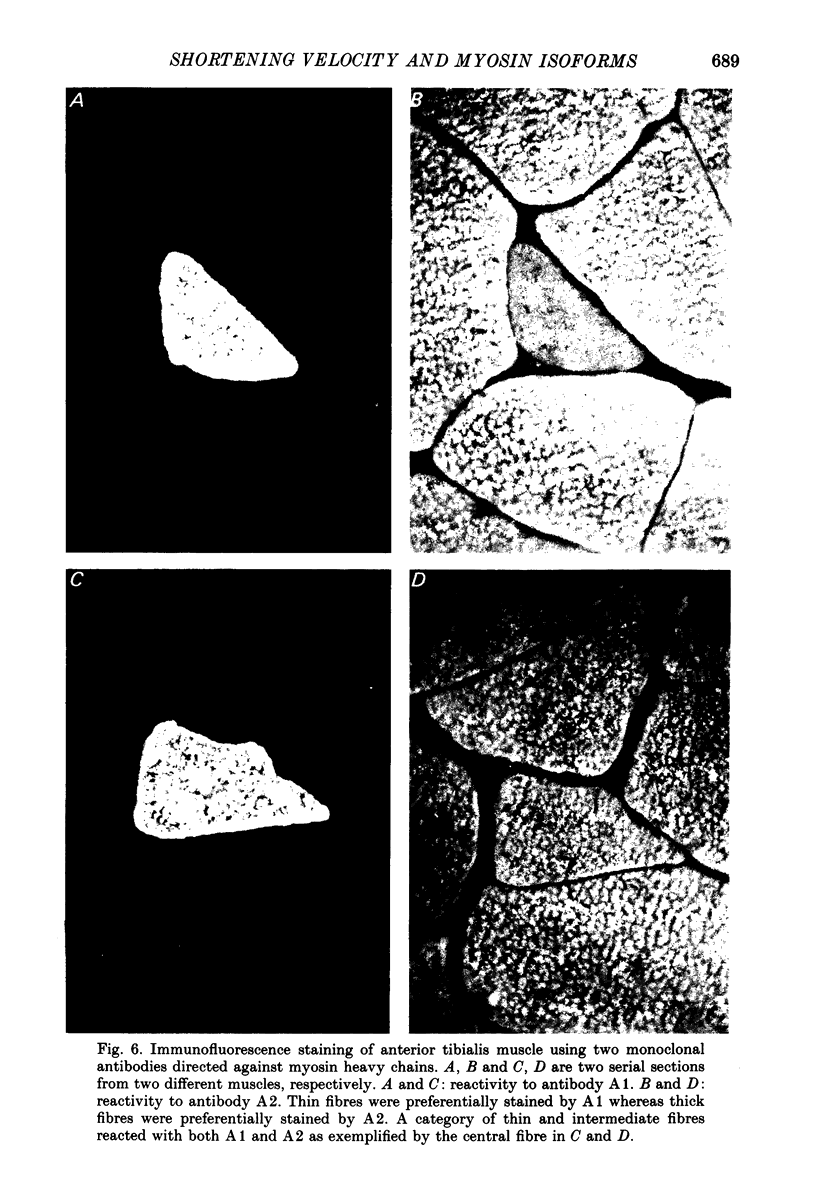
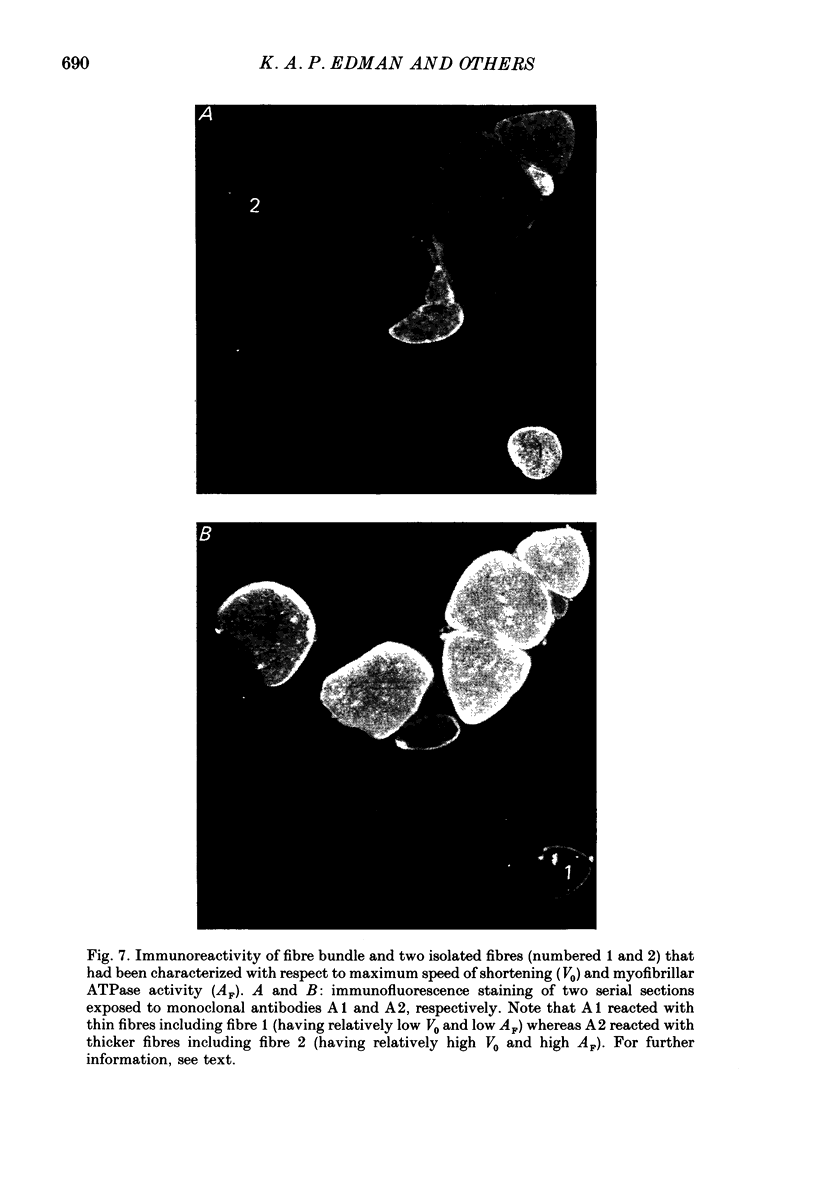
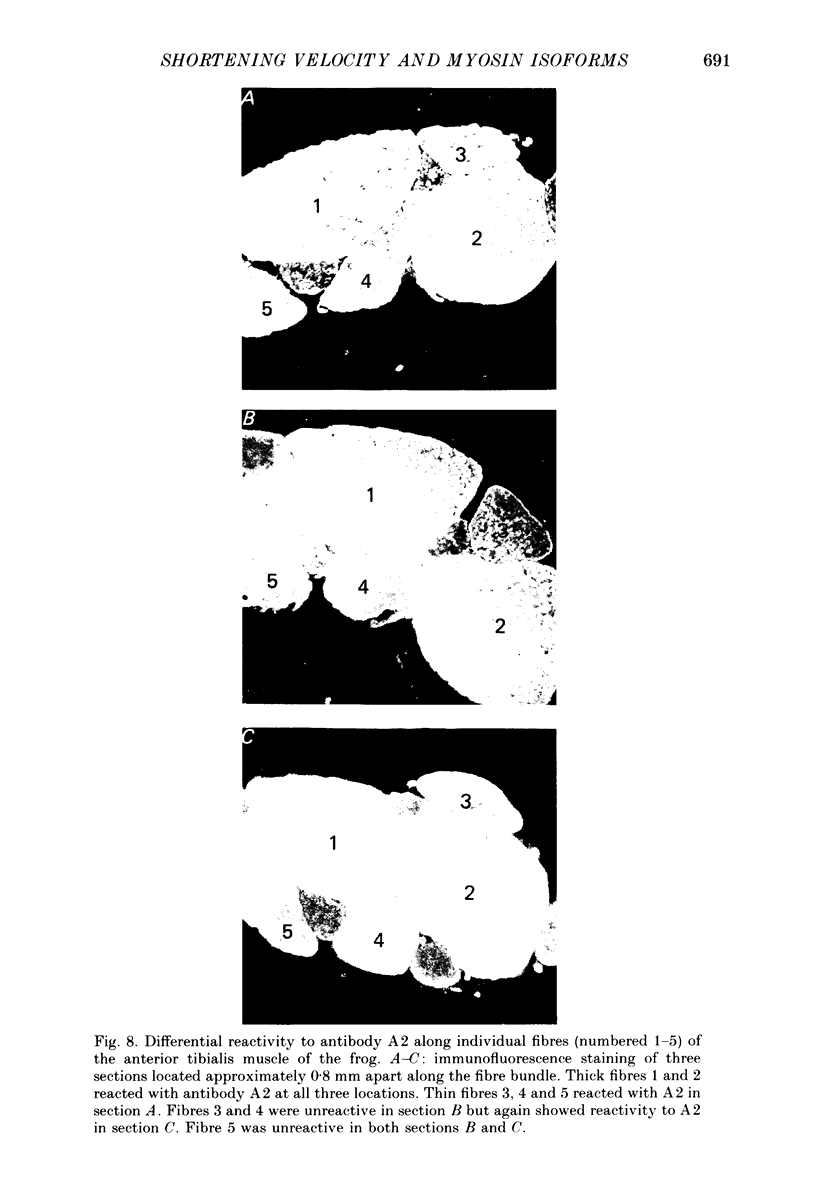
1. The velocity of unloaded shortening (V0), the myofibrillar ATPase activity and the immunoreactivity to two monoclonal antibodies (A1 and A2) that were raised against the myosin heavy chains were studied in single fibres of the anterior tibialis muscle of Rana temporaria. V0 was recorded for the fibre as a whole using the slack-test method. Myofibrillar ATPase activity was determined by means of a quantitative histochemical technique. 2. A highly significant, direct relationship was found to exist between V0 and the myofibrillar ATPase activity recorded in the same single fibres. Both V0 and the myofibrillar ATPase activity changed in proportion to the cross-sectional area of the fibres. 3. Muscle fibres that had first been characterized with respect to V0 and myofibrillar ATPase activity were exposed to monoclonal antibodies A1 and A2. Thin fibres, having relatively low V0 and low myofibrillar ATPase activity, reacted preferentially with A1. Thick fibres, on the other hand, exhibiting relatively high V0 and high myofibrillar ATPase activity, were preferentially stained by A2. A third category of fibres reacted with both A1 and A2. The results support the view that the variability in shortening velocity and myofibrillar ATPase activity that exists among twitch fibres in frog skeletal muscle is based on differences in myosin heavy-chain composition. 4. Attempts were made to elucidate further the previous observation (Edman, Reggiani & te Kronnie, 1985) that the velocity of unloaded shortening (V0) differs along the length of individual muscle fibres. To this end discrete segments (0.5-0.7 mm in length) of intact fibres were delineated by opaque markers of hair that were placed on the fibre surface. The change in length between two adjacent markers (one segment) was recorded photo-electrically while the fibre was released to shorten against a very small load between 2.2 and 2.0 micron sarcomere lengths. In the majority of fibres (eight out of eleven preparations), V0 and myofibrillar ATPase activity exhibited similar patterns of variation along the fibre. Pooled data from thirty-three segments of twelve fibres showed a positive correlation between V0 and myofibrillar ATPase activity (P less than 0.05). 5. The possibility was explored that the myosin isoform composition might vary along the length of an individual muscle fibre. For this purpose bundles of fibres were cross-sectioned at 0.5-1 mm intervals along their entire length and the reactivity to monoclonal antibody A2 was tested at each location.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





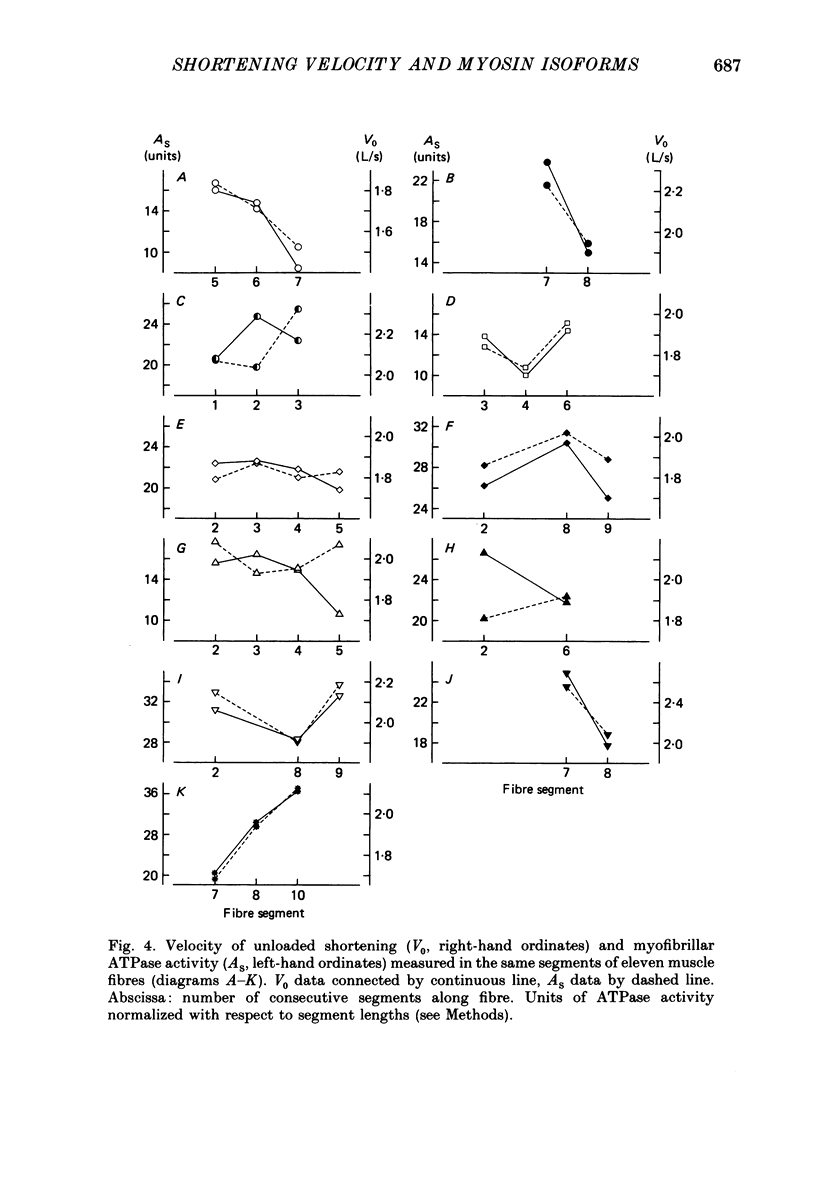
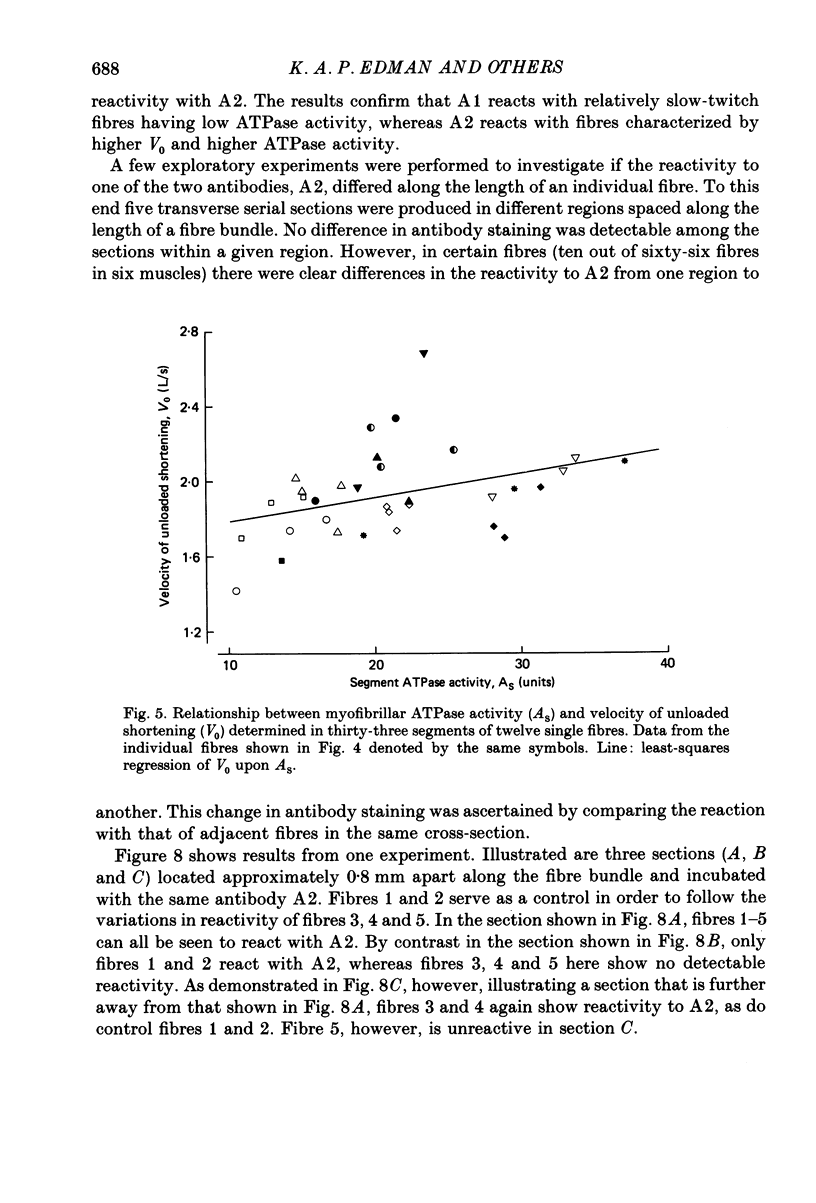



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol. 1984 Jun;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C., te Kronnie G. Differences in maximum velocity of shortening along single muscle fibres of the frog. J Physiol. 1985 Aug;365:147–163. doi: 10.1113/jphysiol.1985.sp015764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Lännergren J. An intermediate type of muscle fibre in Xenopus laevis. Nature. 1979 May 17;279(5710):254–256. doi: 10.1038/279254a0. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Hoh J. F. Myosin isoenzymes in single muscle fibres of Xenopus laevis: analysis of five different functional types. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):401–408. doi: 10.1098/rspb.1984.0072. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Samuel J. L., Rappaport L., Mercadier J. J., Lompre A. M., Sartore S., Triban C., Schiaffino S., Schwartz K. Distribution of myosin isozymes within single cardiac cells. An immunohistochemical study. Circ Res. 1983 Feb;52(2):200–209. doi: 10.1161/01.res.52.2.200. [DOI] [PubMed] [Google Scholar]
- Wagner P. D. Formation and characterization of myosin hybrids containing essential light chains and heavy chains from different muscle myosins. J Biol Chem. 1981 Mar 10;256(5):2493–2498. [PubMed] [Google Scholar]
- Wieczorek D. F., Periasamy M., Butler-Browne G. S., Whalen R. G., Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985 Aug;101(2):618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laarse W. J., Diegenbach P. C., Maslam S. Quantitative histochemistry of three mouse hind-limb muscles: the relationship between calcium-stimulated myofibrillar ATPase and succinate dehydrogenase activities. Histochem J. 1984 May;16(5):529–541. doi: 10.1007/BF01041353. [DOI] [PubMed] [Google Scholar]