Abstract
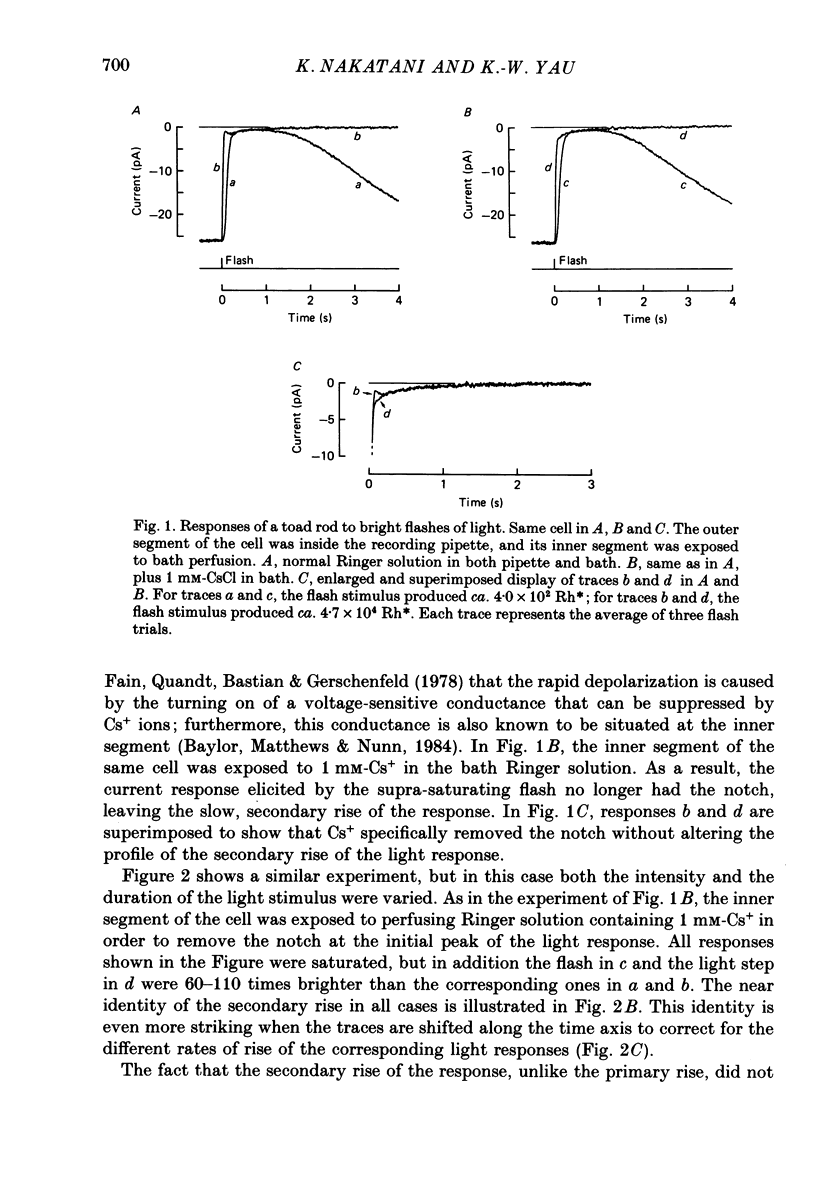
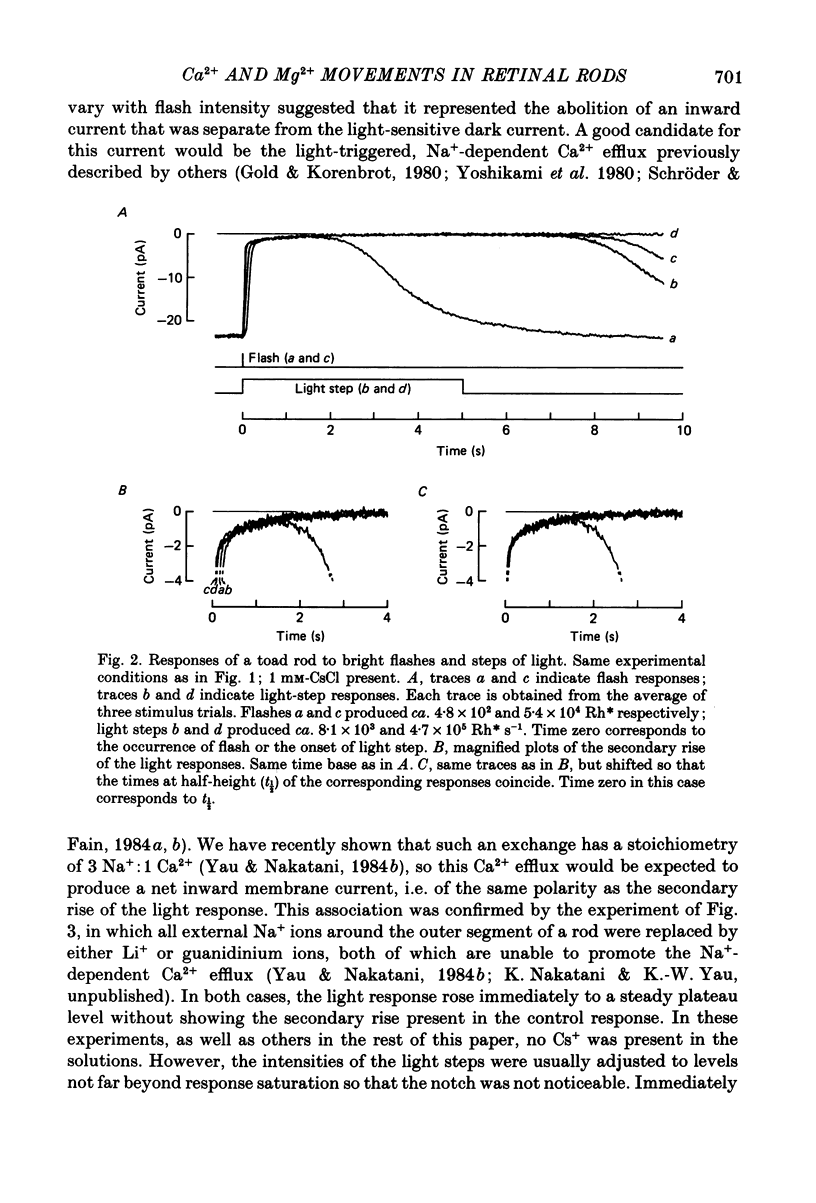
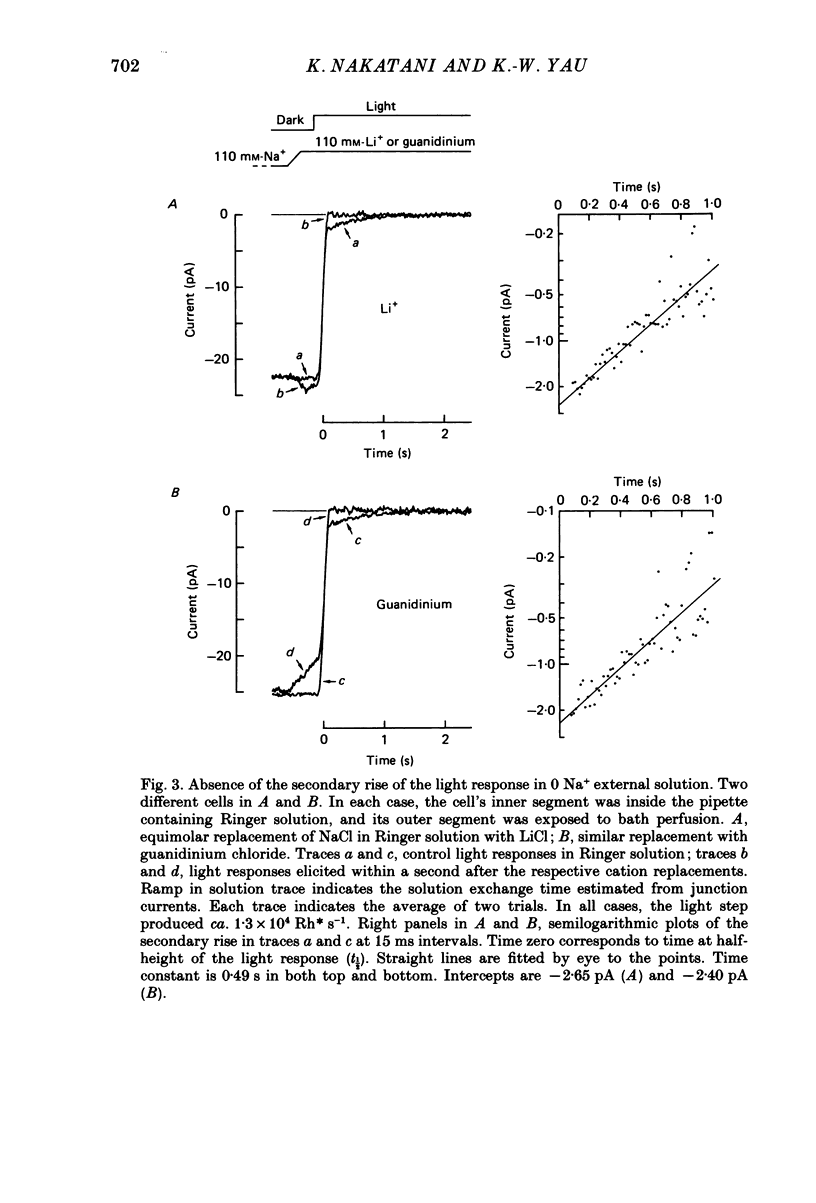
1. Membrane current was recorded from an isolated, dark-adapted toad rod by sucking either its inner segment or outer segment into a tight-fitting glass pipette containing Ringer solution. The remainder of the cell was exposed to bath solution which could be changed rapidly. 2. In normal Ringer solution the current response of a cell to a saturating flash or step of light showed a small secondary rise at its initial peak. The profile of this secondary rise (i.e. amplitude and time course) was independent of both the intensity and the duration of illumination once the light response had reached a plateau level. 3. This secondary rise disappeared when external Na+ around the outer segment was replaced by Li+ or guanidinium, suggesting that it represented an electrogenic Na+-dependent Ca2+ efflux which was declining after the onset of light. 4. This Na+-Ca2+ exchange activity showed a roughly exponential decline, with a time constant of about 0.5 s. Exponential extrapolation of the exchange current to the time at half-height of the light response gave an initial amplitude of about 2 pA. Using La3+ as a blocker, we did not detect any steady exchange current after the initial exponential decline. 5. An intense flash superposed on a just-saturating steady background light failed to produce any incremental exchange current transient. 6. Our interpretation of the above results is that in darkness there are counterbalancing levels of Ca2+ influx (through the light-sensitive conductance) and efflux (through the Na+-Ca2+ exchange) across the plasma membrane of the rod outer segment. The exchange current transient at the onset of light merely represents the unidirectional Ca2+ efflux which becomes revealed as a result of the stoppage of the Ca2+ influx, rather than a de novo Ca2+ efflux triggered by light. 7. Consistent with this interpretation, a test light delivered soon after a saturating, conditioning light elicited little exchange current, which then gradually recovered to control value with a time course parallel to the restoration of the dark current. Conversely, when the dark current was increased above its physiological level by IBMX (isobutylmethylxanthine) the exchange current transient became larger than control.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




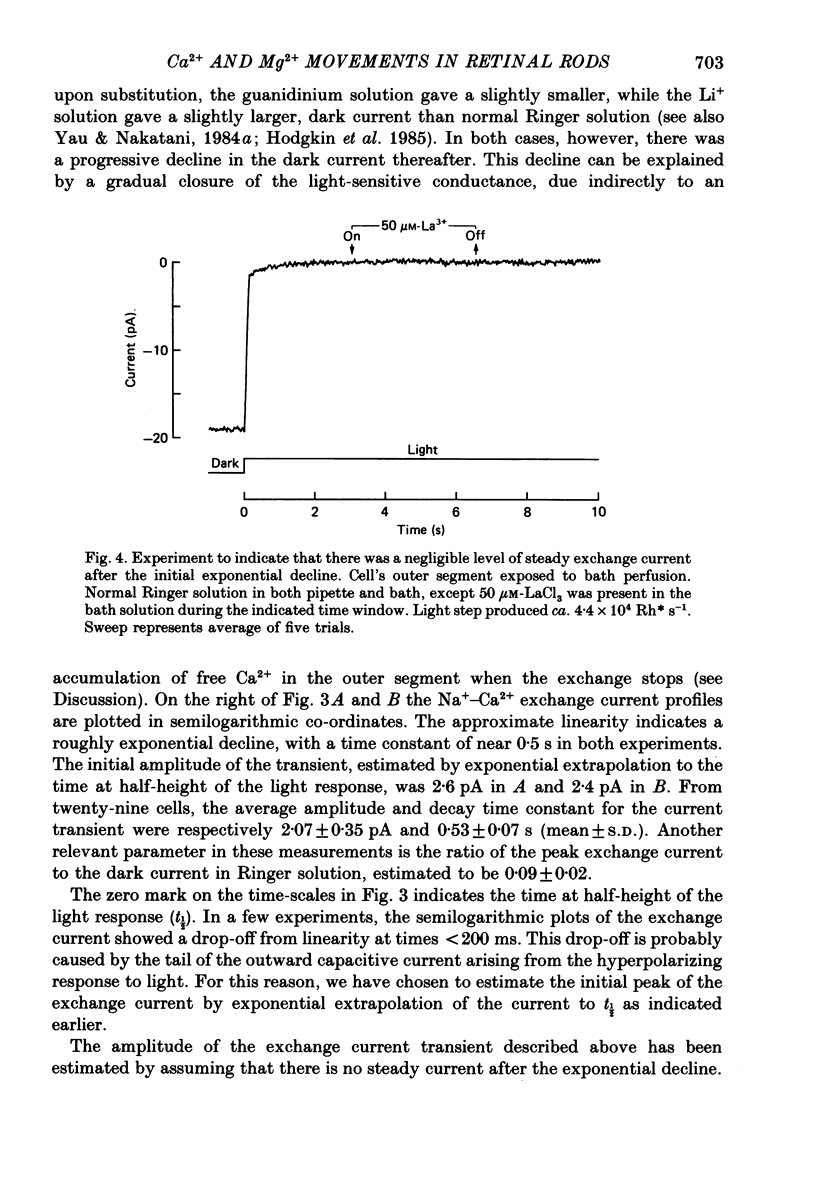
























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., McNaughton P. A. The influence of extracellular calcium binding on the calcium efflux from squid axons. J Physiol. 1978 Mar;276:127–150. doi: 10.1113/jphysiol.1978.sp012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. The effects of sodium replacement on the responses of toad rods. J Physiol. 1982 Sep;330:331–347. doi: 10.1113/jphysiol.1982.sp014344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Nunn B. J. Location and function of voltage-sensitive conductances in retinal rods of the salamander, Ambystoma tigrinum. J Physiol. 1984 Sep;354:203–223. doi: 10.1113/jphysiol.1984.sp015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A., Pinto L. H. Effects of injections of calcium and EGTA into the outer segments of retinal rods of Bufo marinus. J Physiol. 1977 Aug;269(3):707–722. doi: 10.1113/jphysiol.1977.sp011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Caretta A., Cervetto L., Torre V. Ionic movements through light-sensitive channels of toad rods. J Physiol. 1983 Oct;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Antagonism between steady light and phosphodiesterase inhibitors on the kinetics of rod photoresponses. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6698–6702. doi: 10.1073/pnas.79.21.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaggioni A., Sorbi R. T., Turini S. Efflux of potassium from the isolated frog retina: a study of the photic effect. J Physiol. 1972 Apr;222(2):427–445. doi: 10.1113/jphysiol.1972.sp009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P. A. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986 Jan;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Membrane conductances of photoreceptors. Prog Biophys Mol Biol. 1981;37(2):91–147. doi: 10.1016/0079-6107(82)90021-9. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Schröder W. H. Calcium content and calcium exchange in dark-adapted toad rods. J Physiol. 1985 Nov;368:641–665. doi: 10.1113/jphysiol.1985.sp015881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gold G. H., Korenbrot J. I. Light-induced calcium release by intact retinal rods. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5557–5561. doi: 10.1073/pnas.77.9.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G. H. Plasma membrane calcium fluxes in intact rods are inconsistent with the "calcium hypothesis". Proc Natl Acad Sci U S A. 1986 Feb;83(4):1150–1154. doi: 10.1073/pnas.83.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Proceedings: A role for Ca2+ in excitation of retinal rods and cones. Exp Eye Res. 1974 Mar;18(3):299–305. doi: 10.1016/0014-4835(74)90157-2. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Rasmussen H., Dowling J. E. Electrical and adaptive properties of rod photoreceptors in Bufo marinus. II. Effects of cyclic nucleotides and prostaglandins. J Gen Physiol. 1977 Dec;70(6):771–791. doi: 10.1085/jgp.70.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeish P. R., Schwartz E. A., Tachibana M. Control of the generator current in solitary rods of the Ambystoma tigrinum retina. J Physiol. 1984 Mar;348:645–664. doi: 10.1113/jphysiol.1984.sp015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Pinto L. H. Modulation of membrane conductance in rods of Bufo marinus by intracellular calcium ion. J Physiol. 1983 Jun;339:273–298. doi: 10.1113/jphysiol.1983.sp014716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Cobbs W. H. Visual transduction in vertebrate rods and cones: a tale of two transmitters, calcium and cyclic GMP. Vision Res. 1986;26(10):1613–1643. doi: 10.1016/0042-6989(86)90051-9. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Sodium-calcium exchange in the outer segments of bovine rod photoreceptors. J Physiol. 1986 Apr;373:25–45. doi: 10.1113/jphysiol.1986.sp016033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder W. H., Fain G. L. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984 May 17;309(5965):268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Walz B. Elemental distribution in Rana pipiens retinal rods: quantitative electron probe analysis. J Physiol. 1985 Jan;358:183–195. doi: 10.1113/jphysiol.1985.sp015547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical activity of vertebrate photoreceptors. Q Rev Biophys. 1970 May;3(2):179–222. doi: 10.1017/s0033583500004571. [DOI] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V. The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol. 1982 Dec;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M. L., Fain G. L., Bastian B. L. Light-dependent ion influx into toad photoreceptors. J Gen Physiol. 1982 Oct;80(4):517–536. doi: 10.1085/jgp.80.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Lamb T. D., Baylor D. A. Light-induced fluctuations in membrane current of single toad rod outer segments. Nature. 1977 Sep 1;269(5623):78–80. doi: 10.1038/269078a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., George J. S., Hagins W. A. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980 Jul 24;286(5771):395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]




