Abstract
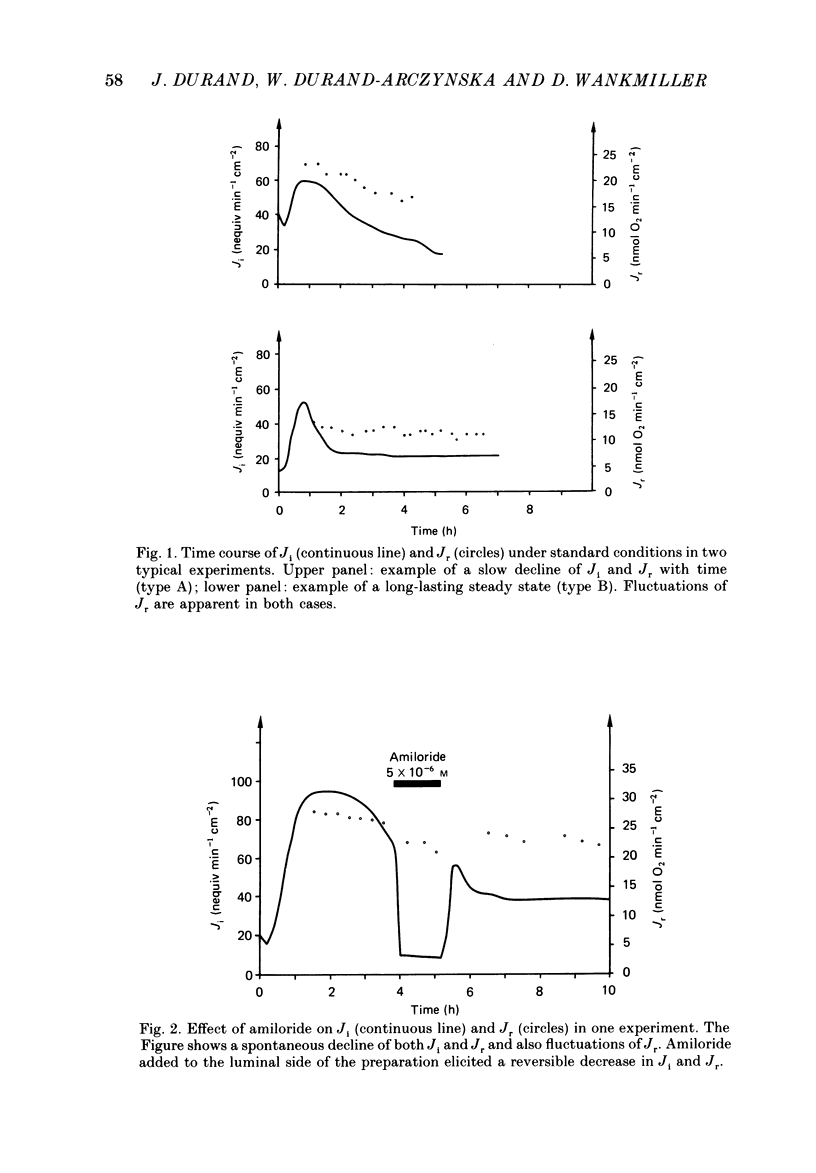
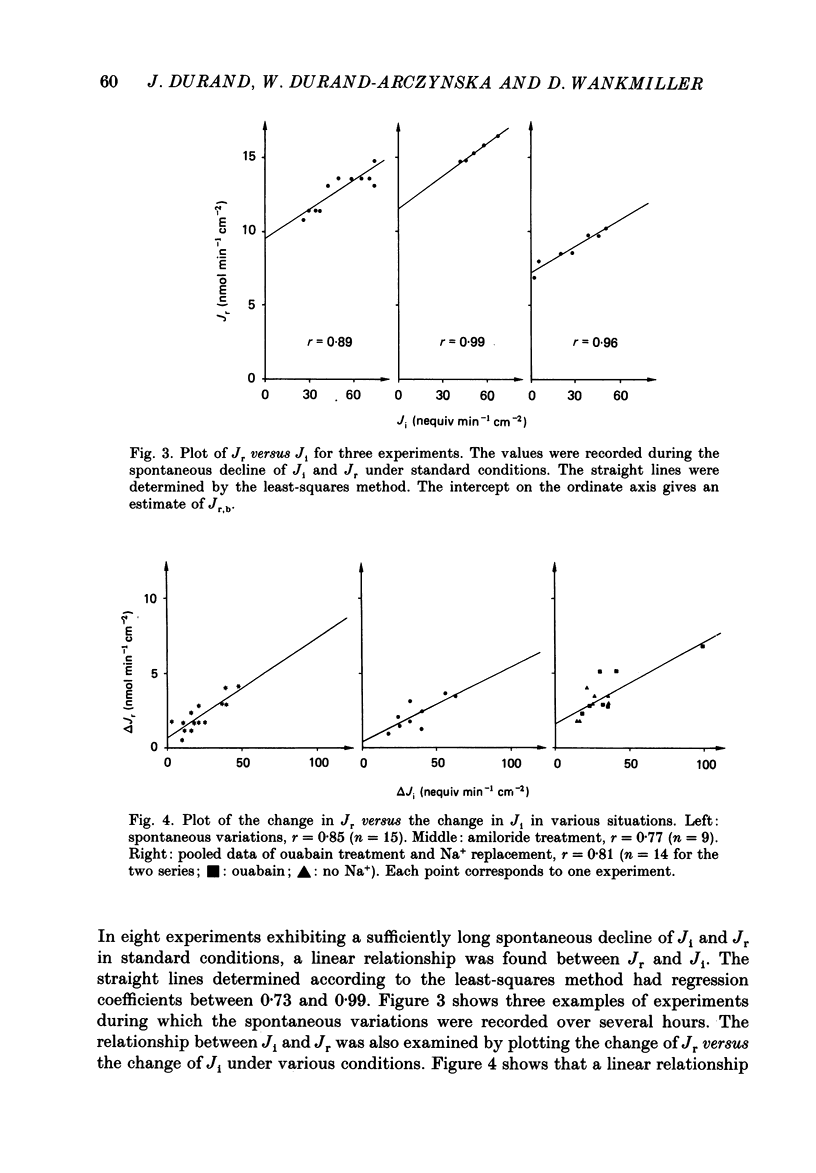
1. Active transepithelial Na+ transport (Ji) and O2 consumption (Jr) were measured simultaneously in rabbit distal colon, under standard (control) incubation conditions and after various manoeuvres, known to inhibit Na+ transport. 2. The determination of Jr was complicated by the presence of fluctuations of the PO2 in the incubation solution and by spontaneous variations of the tissue respiration, which usually declined slowly with time. 3. The control values of Ji and Jr after 2 h incubation were 55 +/- 4 nequiv min-1 cm-2 and 16 +/- 1 nmol O2 min-1 cm-2, respectively (n = 44). The electrical resistance was 386 +/- 23 omega cm2; it was stable over 6 h. 4. Ji was reduced to a very low level with either amiloride, ouabain or Na+ substitution with choline. In all instances, Jr decreased concomitantly by 15-30%. 5. A plot of the change in Jr versus the change in Ji gave a straight line for all situations, i.e. for the spontaneous decline of Na+ transport and respiration and for the effects of the inhibitors. 6. The linearity between Jr and Ji allows for the determination of a stoichiometric ratio. It is of similar magnitude, when calculated either with the data of spontaneous variations or with those obtained by the action of any inhibitor tested. It is 15-20 Na+ ions per O2 molecule, a value close to that reported previously for amphibian epithelia and also close to the maximum theoretical value of 18 Na+ ions per O2 molecule.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clauss W., Schäfer H., Horch I., Hörnicke H. Segmental differences in electrical properties and Na-transport of rabbit caecum, proximal and distal colon in vitro. Pflugers Arch. 1985 Mar;403(3):278–282. doi: 10.1007/BF00583600. [DOI] [PubMed] [Google Scholar]
- Durand J., Durand-Arczynska W., Schoenenweid F. Oxygen consumption and active sodium and chloride transport in bovine tracheal epithelium. J Physiol. 1986 Mar;372:51–62. doi: 10.1113/jphysiol.1986.sp015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJIMOTO M., NASH F. D., KESSLER R. H. EFFECTS OF CYANIDE, QO, AND DINITROPHENOL ON RENAL SODIUM REABSORPTION AND OXYGEN CONSUMPTION. Am J Physiol. 1964 Jun;206:1327–1332. doi: 10.1152/ajplegacy.1964.206.6.1327. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Frizzell R. A. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. Am J Physiol. 1986 Aug;251(2 Pt 1):C252–C267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Heintze K., Stewart C. P., Frizzell R. A. Sodium-dependent chloride secretion across rabbit descending colon. Am J Physiol. 1983 Apr;244(4):G357–G365. doi: 10.1152/ajpgi.1983.244.4.G357. [DOI] [PubMed] [Google Scholar]
- KIIL F., AUKLAND K., REFSUM H. E. Renal sodium transport and oxygen consumption. Am J Physiol. 1961 Sep;201:511–516. doi: 10.1152/ajplegacy.1961.201.3.511. [DOI] [PubMed] [Google Scholar]
- Kiil F., Sejersted O. M., Steen P. A. Energetics and specificity of transcellular NaCl transport in the dog kidney. Int J Biochem. 1980;12(1-2):245–250. doi: 10.1016/0020-711x(80)90079-8. [DOI] [PubMed] [Google Scholar]
- LASSEN N. A., MUNCK O., THAYSEN J. H. Oxygen consumption and sodium reabsorption in the kidney. Acta Physiol Scand. 1961 Apr;51:371–384. doi: 10.1111/j.1748-1716.1961.tb02147.x. [DOI] [PubMed] [Google Scholar]
- LASSEN U. V., THAYSEN J. H. Correlation between sodium transport and oxygen consumption in isolated renal tissue. Biochim Biophys Acta. 1961 Mar 4;47:616–618. doi: 10.1016/0006-3002(61)90567-4. [DOI] [PubMed] [Google Scholar]
- LEAF A., PAGE L. B., ANDERSON J. Respiration and active sodium transport of isolated toad bladder. J Biol Chem. 1959 Jun;234(6):1625–1629. [PubMed] [Google Scholar]
- LEAF A., RENSHAW A. Ion transport and respiration of isolated frog skin. Biochem J. 1957 Jan;65(1):82–90. doi: 10.1042/bj0650082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Canessa M., Leaf A. Metabolic cost of sodium transport in toad urinary bladder. J Membr Biol. 1977 Apr 22;32(3-4):383–401. doi: 10.1007/BF01905229. [DOI] [PubMed] [Google Scholar]
- Lang M. A., Caplan S. R., Essig A. Thermodynamic analysis of active sodium transport and oxidative metabolism in toad urinary bladder. J Membr Biol. 1977 Feb 24;31(1-2):19–29. doi: 10.1007/BF01869397. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Balaban R. S. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981 May;240(5):F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- Moran W. M., Hudson R. L., Schultz S. G. Kinetics of the effect of amiloride on the permeability of the apical membrane of rabbit descending colon to sodium. J Membr Biol. 1985;87(1):55–65. doi: 10.1007/BF01870699. [DOI] [PubMed] [Google Scholar]
- Nellans H. N., Finn A. L. Oxygen consumption and sodium transport in the toad urinary bladder. Am J Physiol. 1974 Sep;227(3):670–675. doi: 10.1152/ajplegacy.1974.227.3.670. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. A cellular model for active sodium absorption by mammalian colon. Annu Rev Physiol. 1984;46:435–451. doi: 10.1146/annurev.ph.46.030184.002251. [DOI] [PubMed] [Google Scholar]
- Silverthorn S. U., Eaton D. C. Respiration and sodium transport in rabbit urinary bladder. Biochim Biophys Acta. 1982 Jul 28;689(2):299–308. doi: 10.1016/0005-2736(82)90263-2. [DOI] [PubMed] [Google Scholar]
- Smaje L. H., Poulsen J. H., Ussing H. H. Evidence from O2 uptake measurements for Na+ -K+ -2 Cl- co-transport in the rabbit submandibular gland. Pflugers Arch. 1986 May;406(5):492–496. doi: 10.1007/BF00583372. [DOI] [PubMed] [Google Scholar]
- Smith P. L., McCabe R. D. Mechanism and regulation of transcellular potassium transport by the colon. Am J Physiol. 1984 Nov;247(5 Pt 1):G445–G456. doi: 10.1152/ajpgi.1984.247.5.G445. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. III. The ATP dependence of the Na pump. J Gen Physiol. 1984 Oct;84(4):643–662. doi: 10.1085/jgp.84.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torelli G., Milla E., Faelli A., Costantini S. Energy requirement for sodium reabsorption in the in vivo rabbit kidney. Am J Physiol. 1966 Sep;211(3):576–580. doi: 10.1152/ajplegacy.1966.211.3.576. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WILLIS J. S. ION MOVEMENTS AND OXYGEN CONSUMPTION IN KIDNEY CORTEX SLICES. J Physiol. 1963 Aug;168:158–177. doi: 10.1113/jphysiol.1963.sp007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. Energetics of chloride secretion in canine tracheal epithelium. Comparison of the metabolic cost of chloride transport with the metabolic cost of sodium transport. J Clin Invest. 1984 Jul;74(1):262–268. doi: 10.1172/JCI111410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorio T., Bentley P. J. Permeability of the rabbit colon in vitro. Am J Physiol. 1977 Jan;232(1):F5–F9. doi: 10.1152/ajprenal.1977.232.1.F5. [DOI] [PubMed] [Google Scholar]
- ZERAHN K. Oxygen consumption and active sodium transport in the isolated and short-circuited frog skin. Acta Physiol Scand. 1956 May 31;36(4):300–318. doi: 10.1111/j.1748-1716.1956.tb01327.x. [DOI] [PubMed] [Google Scholar]


