Abstract
1. The intracellular pH (pHi) of live fibres from the anterior tibialis of the frog Rana temporaria was measured at 10 degrees C (using pH-sensitive microelectrodes) in Ringer solutions containing a fixed bicarbonate concentration (20 mM) and varying PCO2 concentrations of 0.5-54%. As extracellular pH was changed from 7.99 to 6.00, mean pHi changed from 7.24 to 5.97. Similar results were obtained at 20 degrees C. 2. In parallel experiments force and rate of heat production in 4 s isometric tetani at 10 degrees C were measured, and compared to control observations (5% CO2, pHi 6.80). 3. As the fibres became more acid (to pHi 5.95), force and heat rate were progressively reduced (to 0.75 and 0.71 of the control values, respectively). 4. As the fibres became more alkaline (to pHi 7.26), force increased slightly (by a maximum of 0.03 of the control value) but heat rate did not increase. 5. When the dependence on pH of the molar enthalpy change for phosphocreatine splitting is taken into account, these results indicate that the force-time integral per cross-bridge cycle increases with pHi over this range.
Full text
PDF

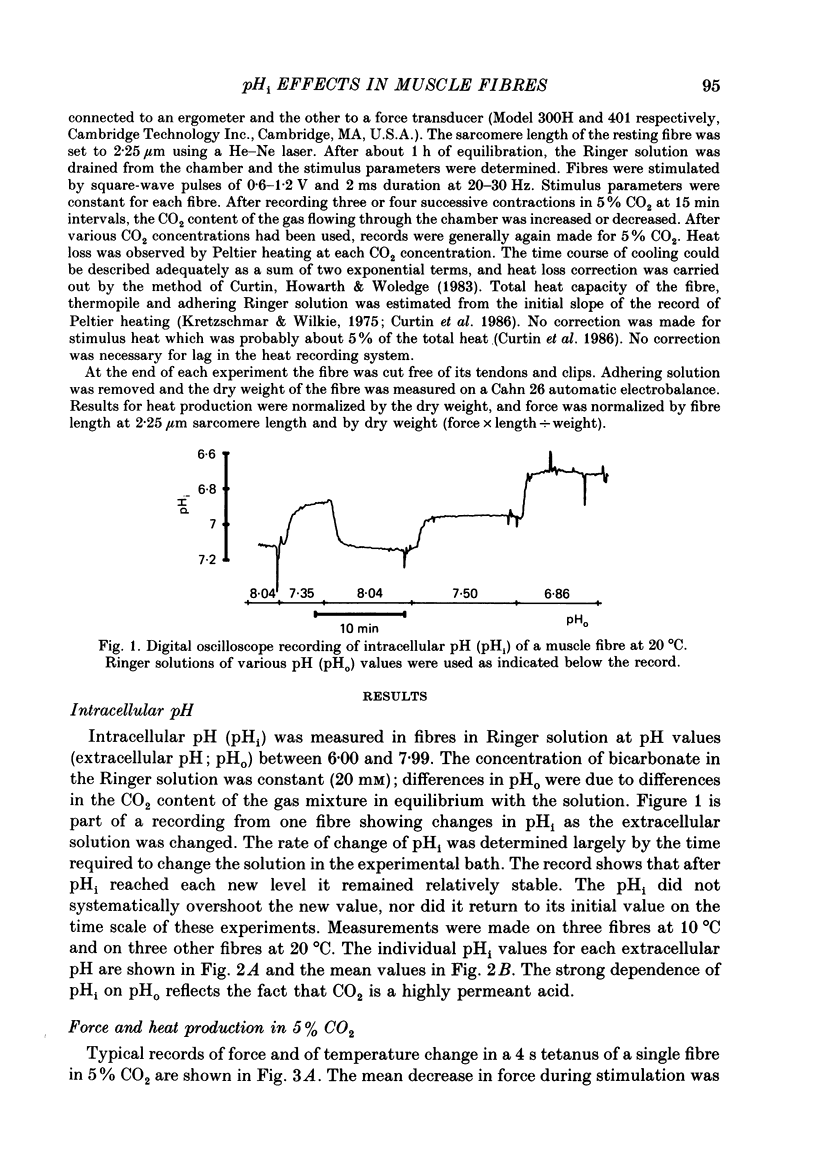
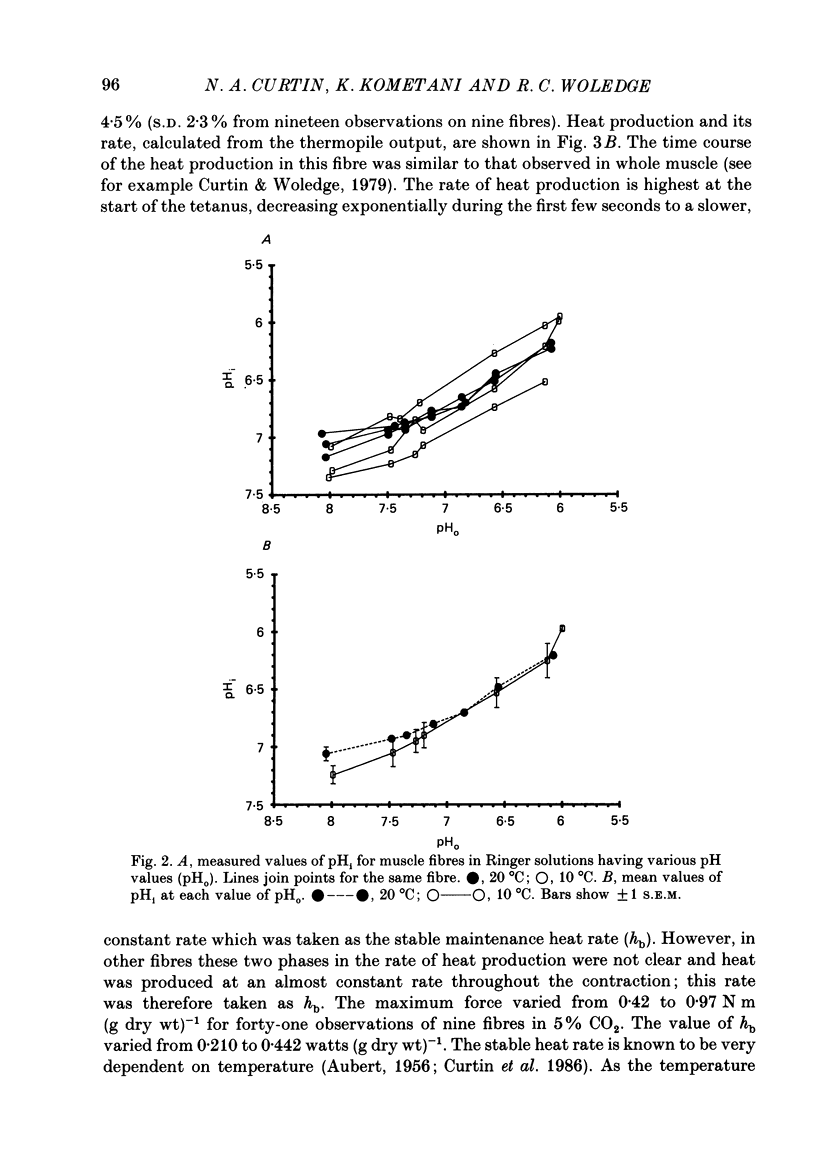
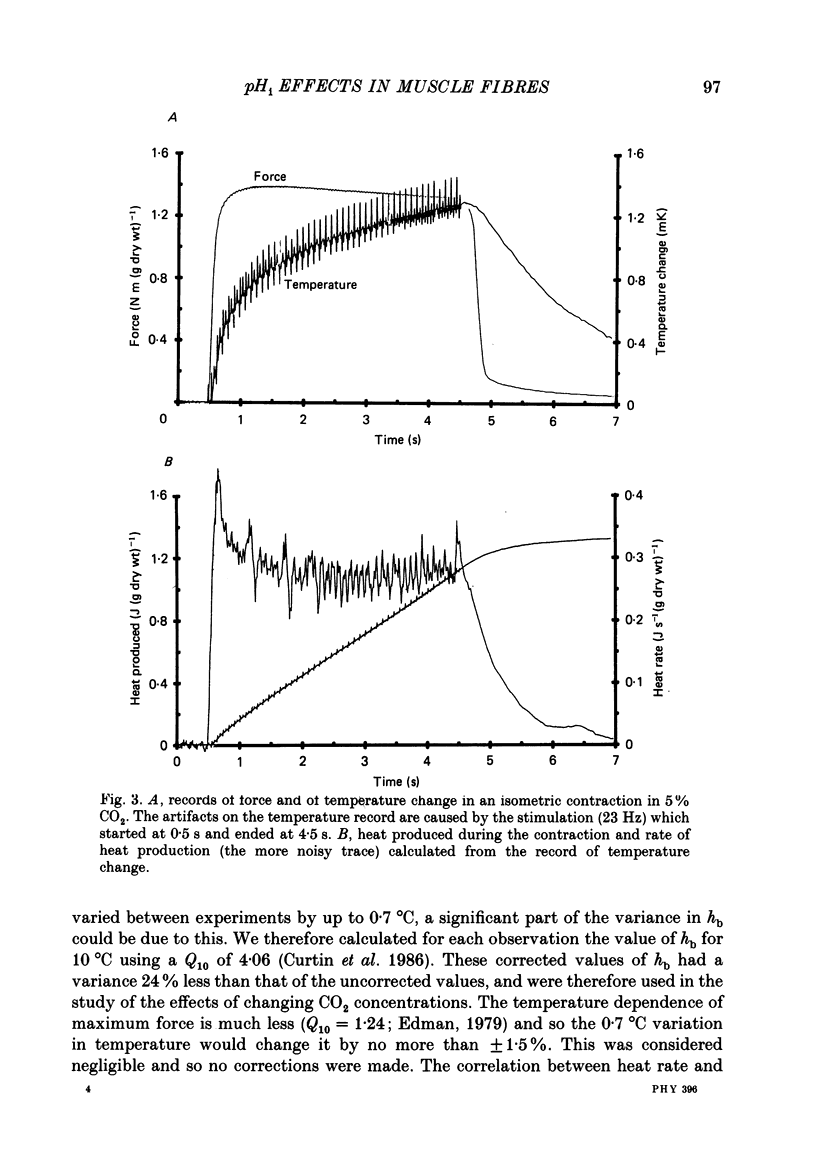
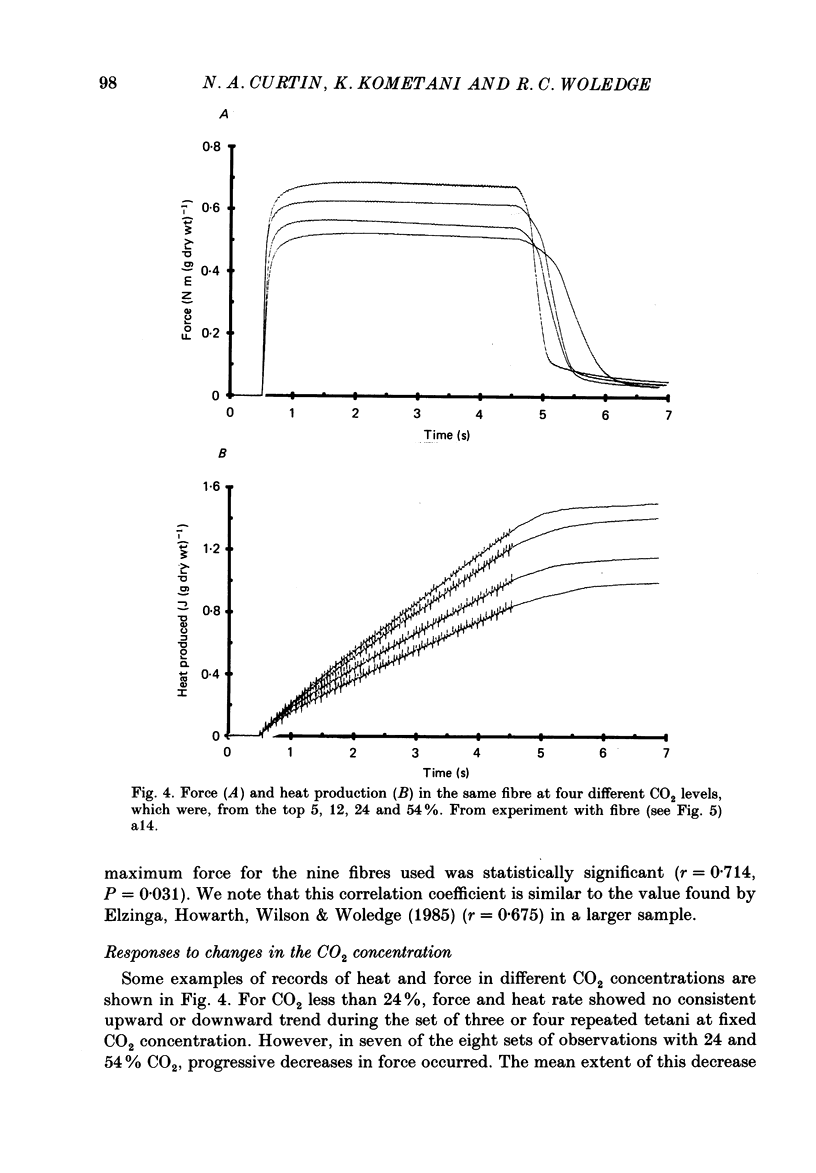
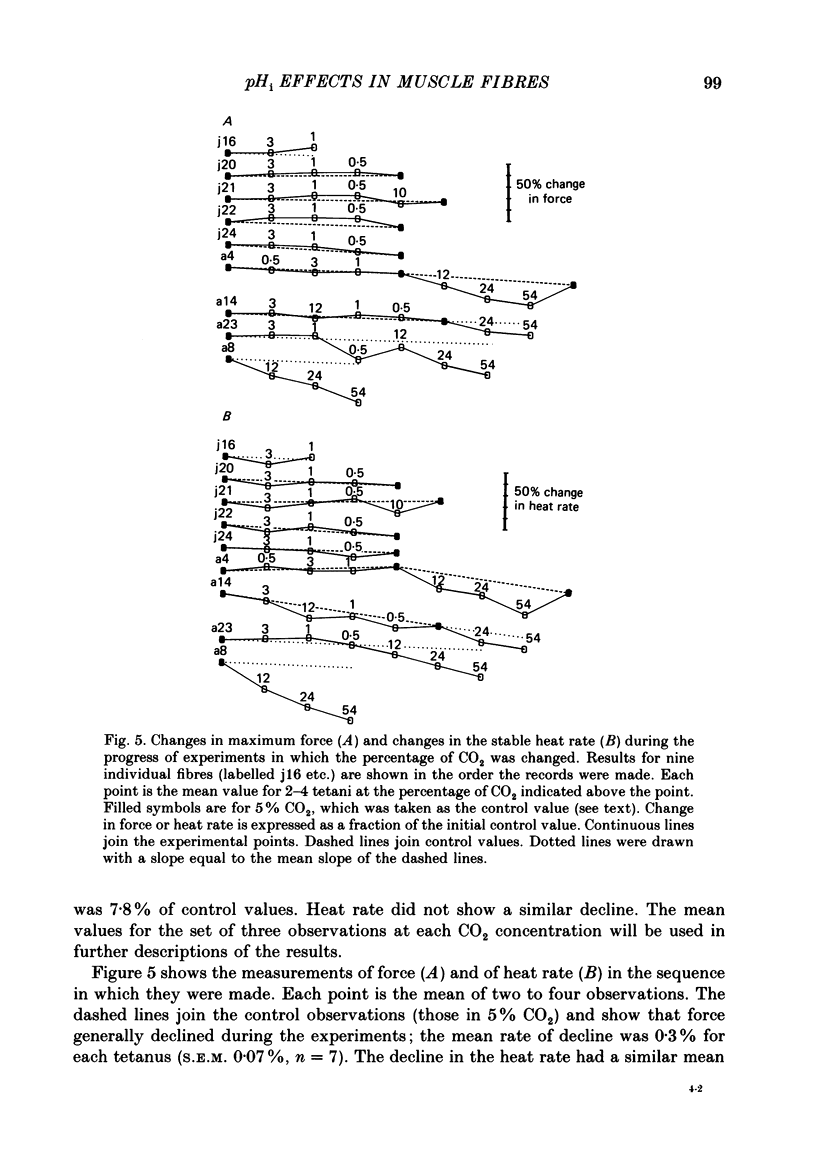
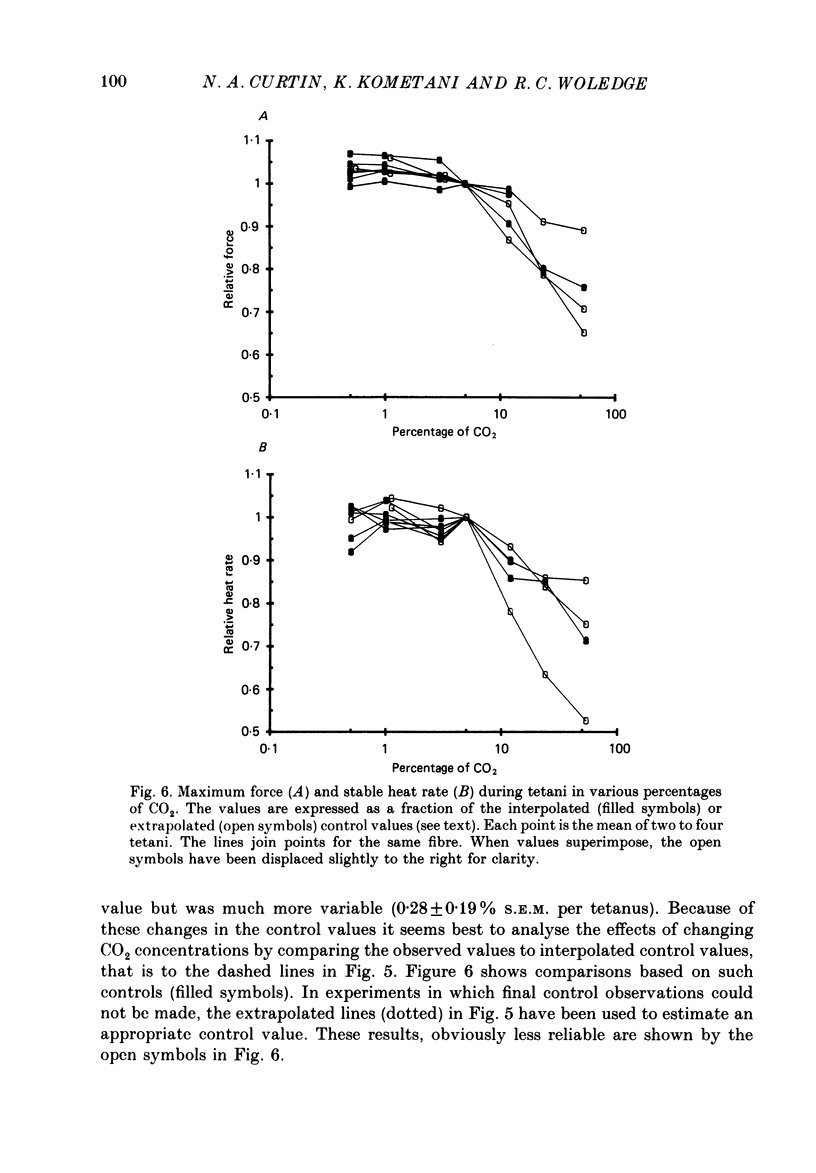
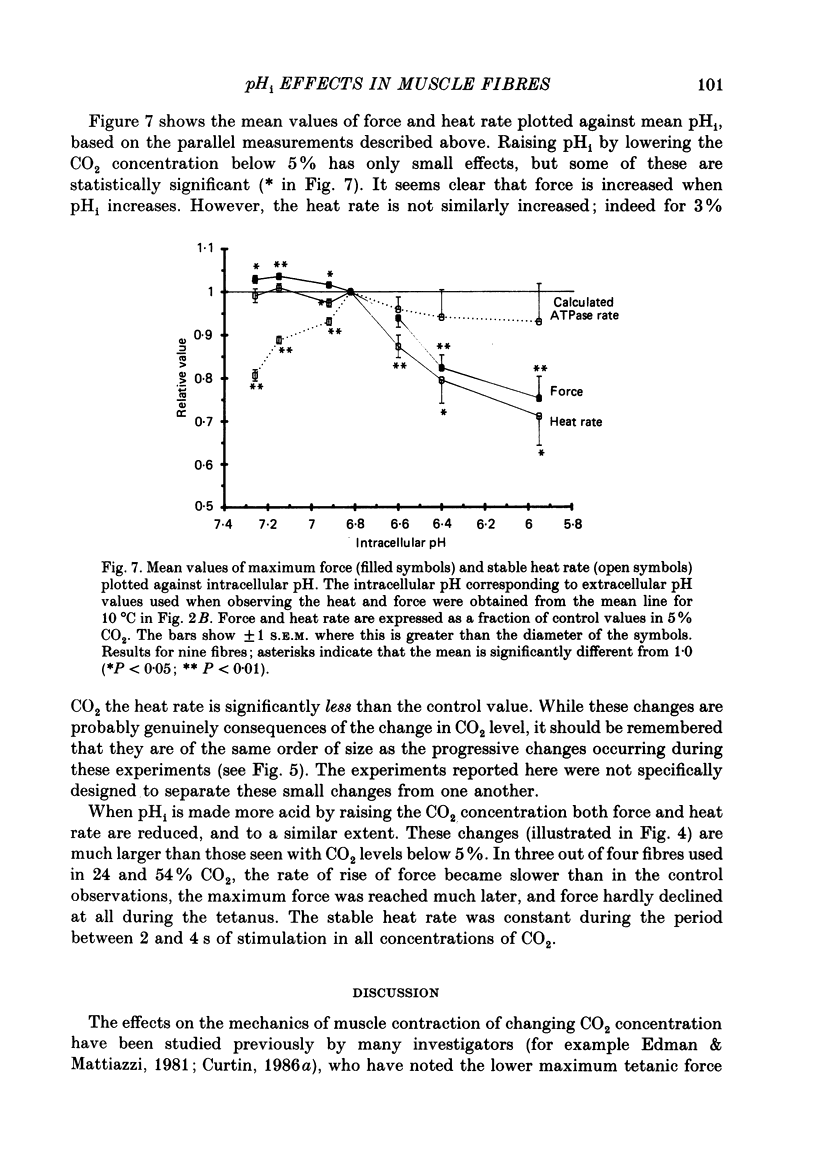



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Putnam R. W., Roos A. The intracellular pH of frog skeletal muscle: its regulation in isotonic solutions. J Physiol. 1983 Dec;345:175–187. doi: 10.1113/jphysiol.1983.sp014973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A. Buffer power and intracellular pH of frog sartorius muscle. Biophys J. 1986 Nov;50(5):837–841. doi: 10.1016/S0006-3495(86)83524-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A. Effects of carbon dioxide and tetanus duration on relaxation of frog skeletal muscle. J Muscle Res Cell Motil. 1986 Jun;7(3):269–275. doi: 10.1007/BF01753560. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Absolute values of myothermic measurements on single muscle fibres from frog. J Muscle Res Cell Motil. 1986 Aug;7(4):327–332. doi: 10.1007/BF01753653. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Woledge R. C. Heat production by single fibres of frog muscle. J Muscle Res Cell Motil. 1983 Apr;4(2):207–222. doi: 10.1007/BF00712031. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Chemical change and energy production during contraction of frog muscle: how are their time courses related? J Physiol. 1979 Mar;288:353–366. [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Effect of muscle length on energy balance in frog skeletal muscle. J Physiol. 1981 Jul;316:453–468. doi: 10.1113/jphysiol.1981.sp013800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Kean C. J., Wallner A., Garibian-Sarian V. The time-course of energy balance in an isometric tetanus. J Gen Physiol. 1979 May;73(5):553–567. doi: 10.1085/jgp.73.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. The use of the Peltier effect for simple and accurate calibration of thermoelectric devices. Proc R Soc Lond B Biol Sci. 1975 Aug 19;190(1100):315–321. doi: 10.1098/rspb.1975.0095. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]


