Abstract
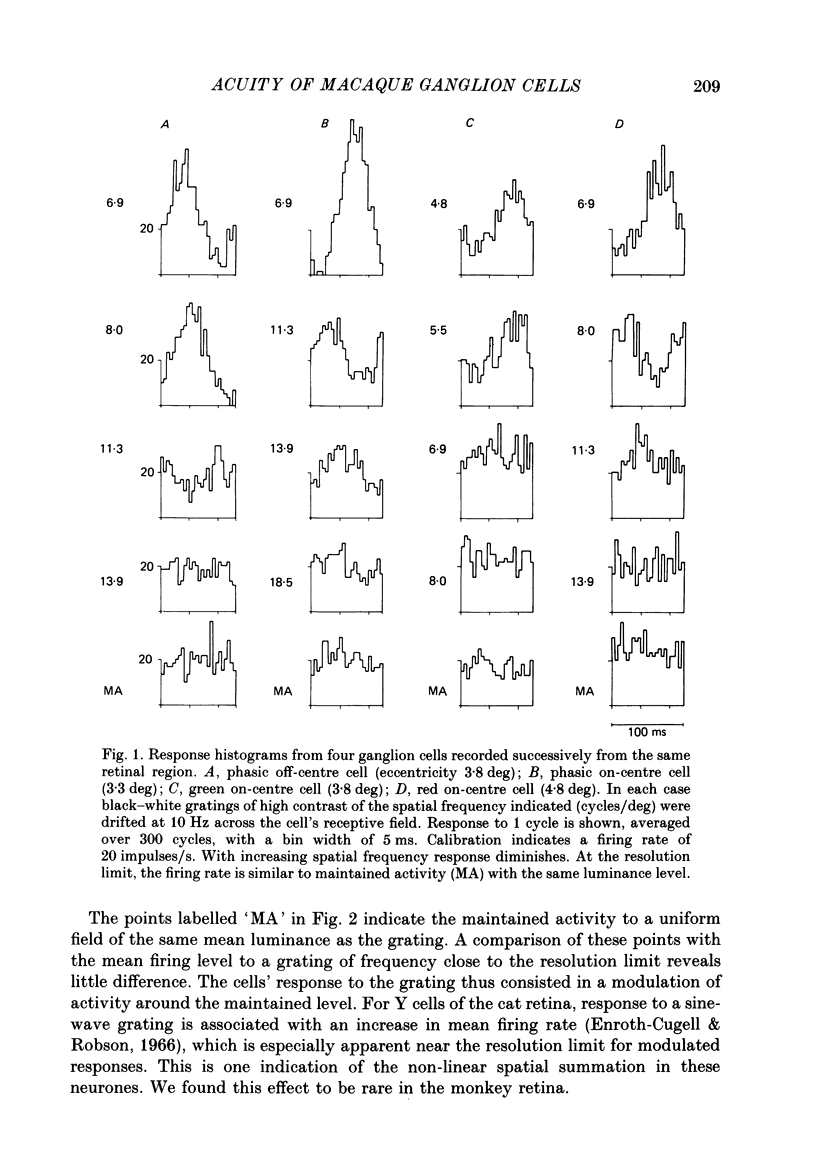
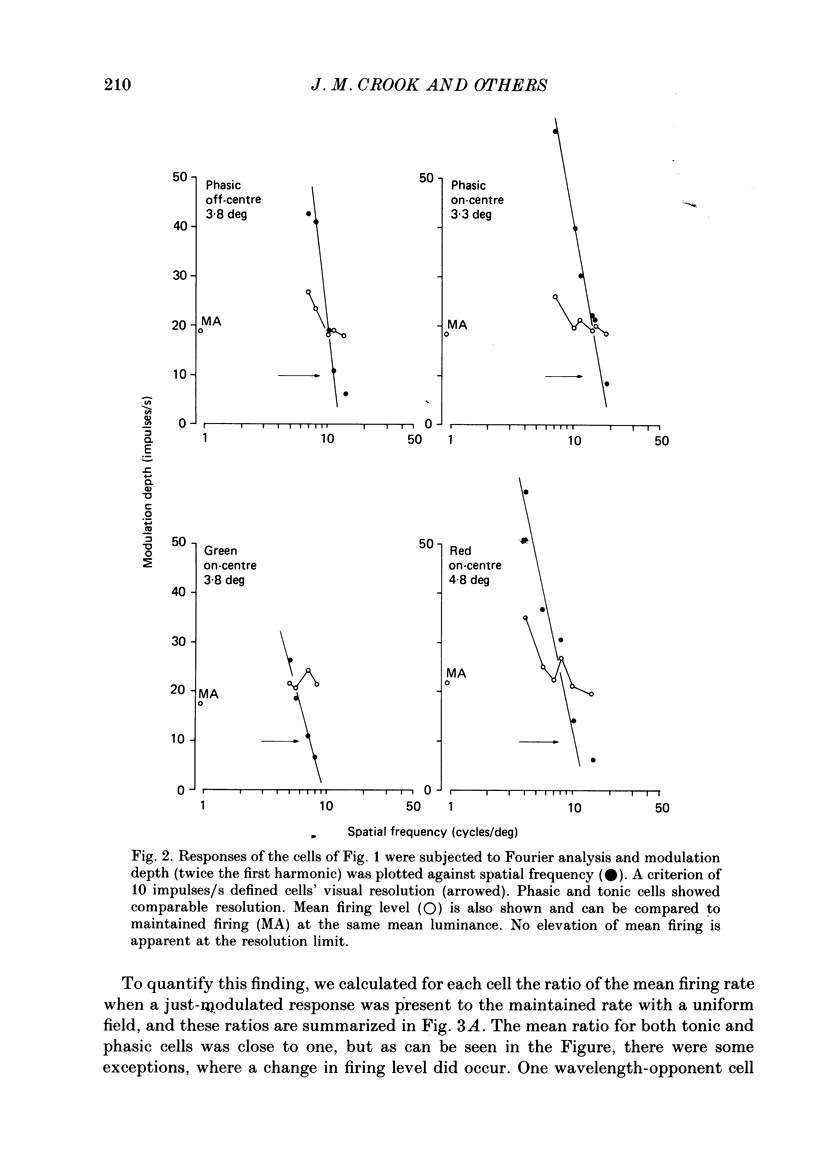
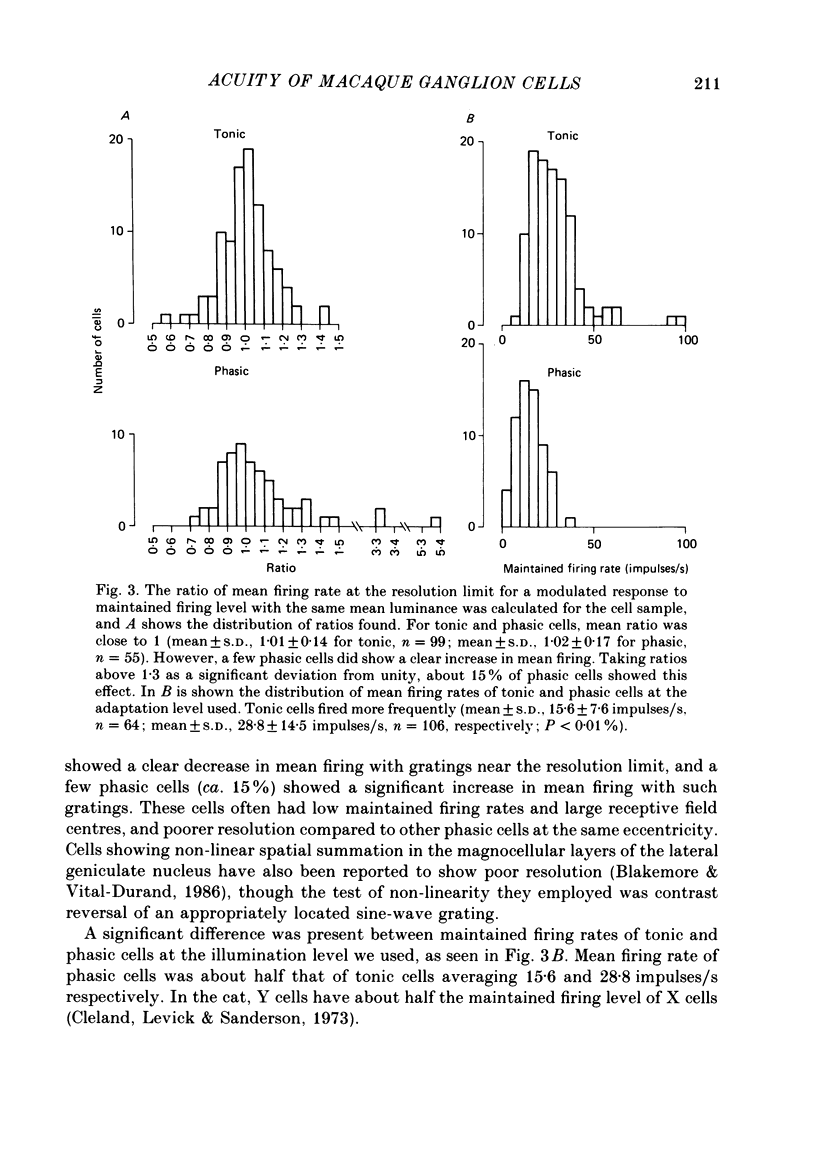
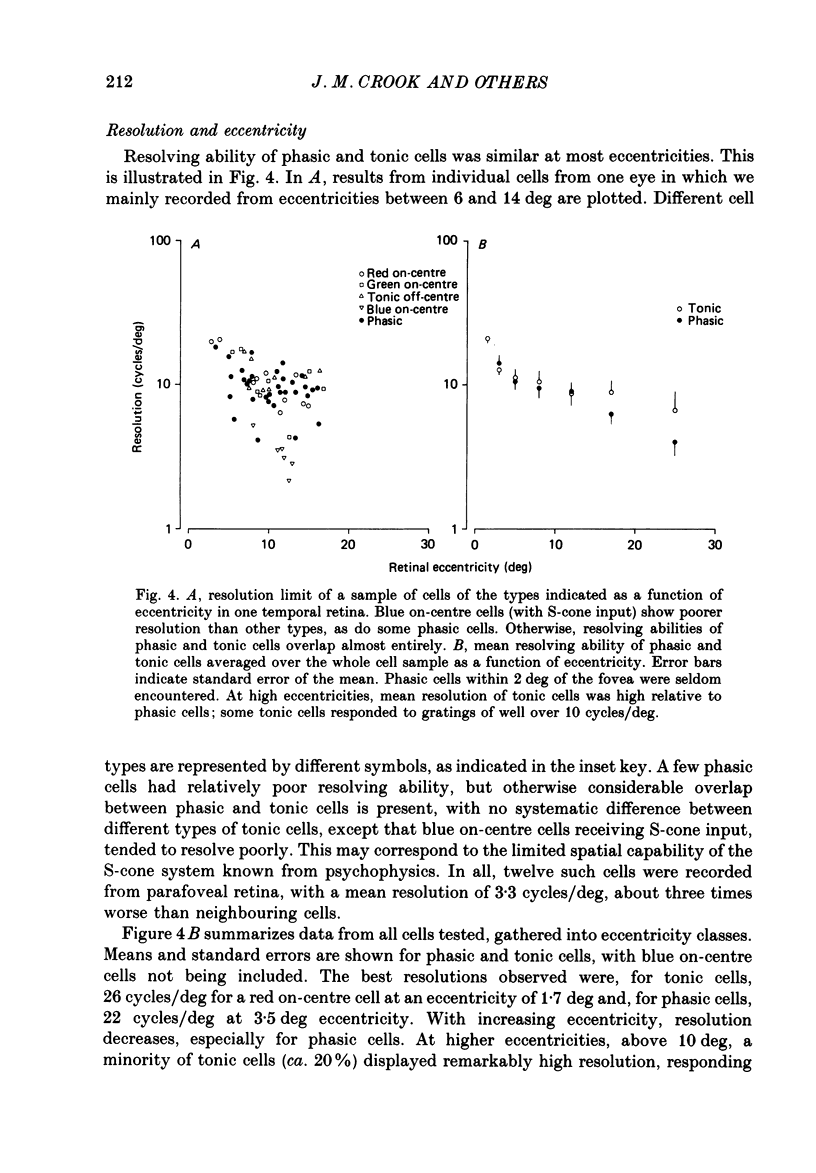
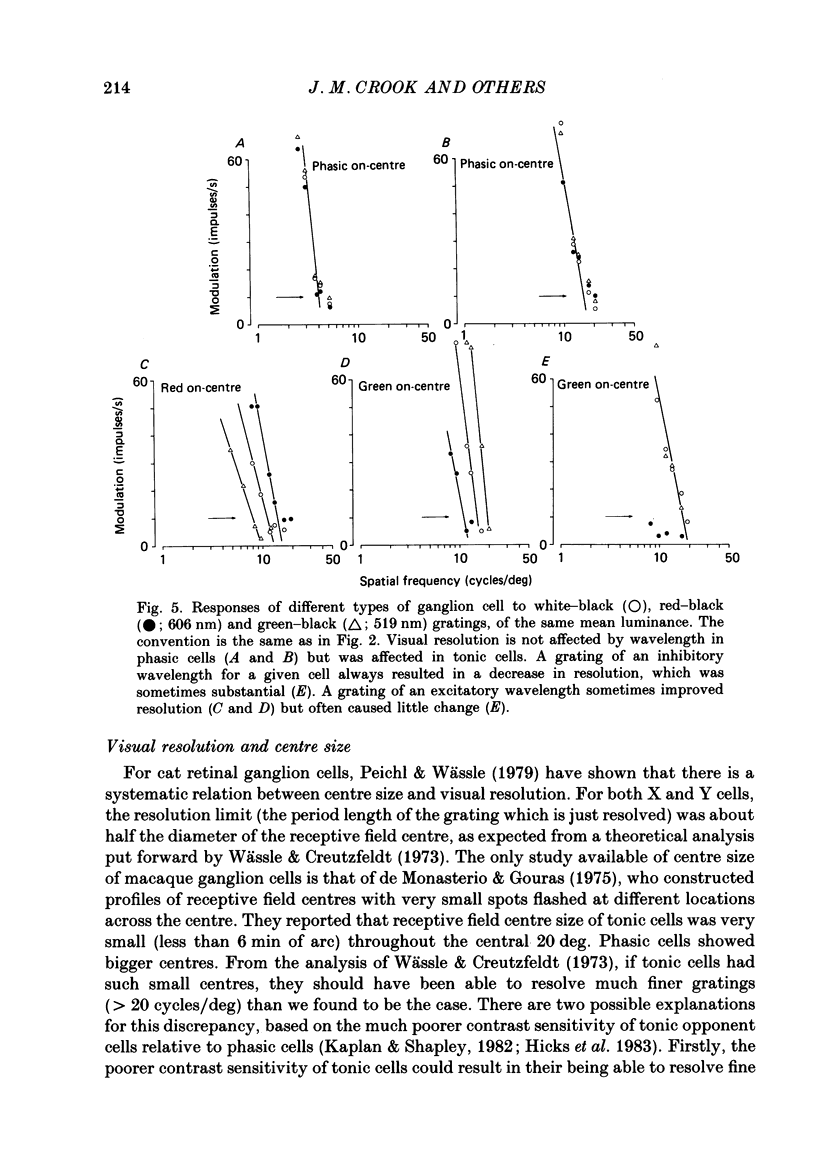
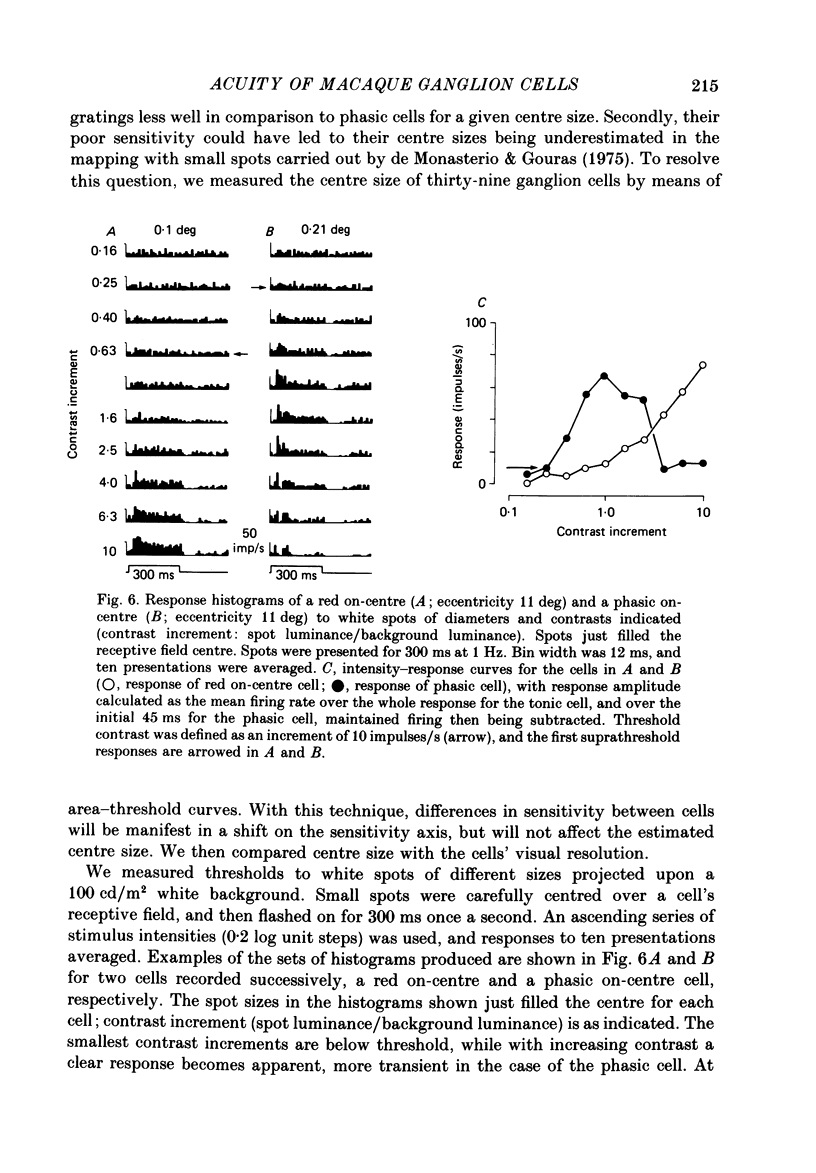
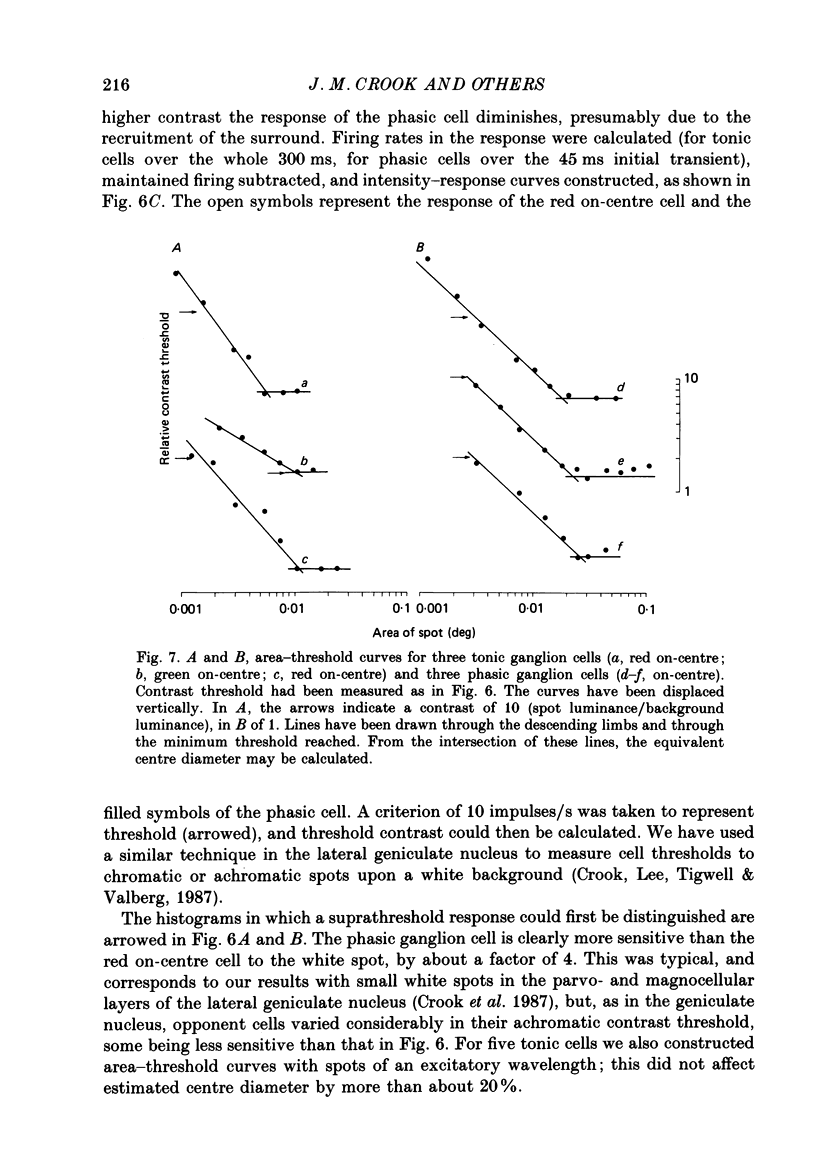
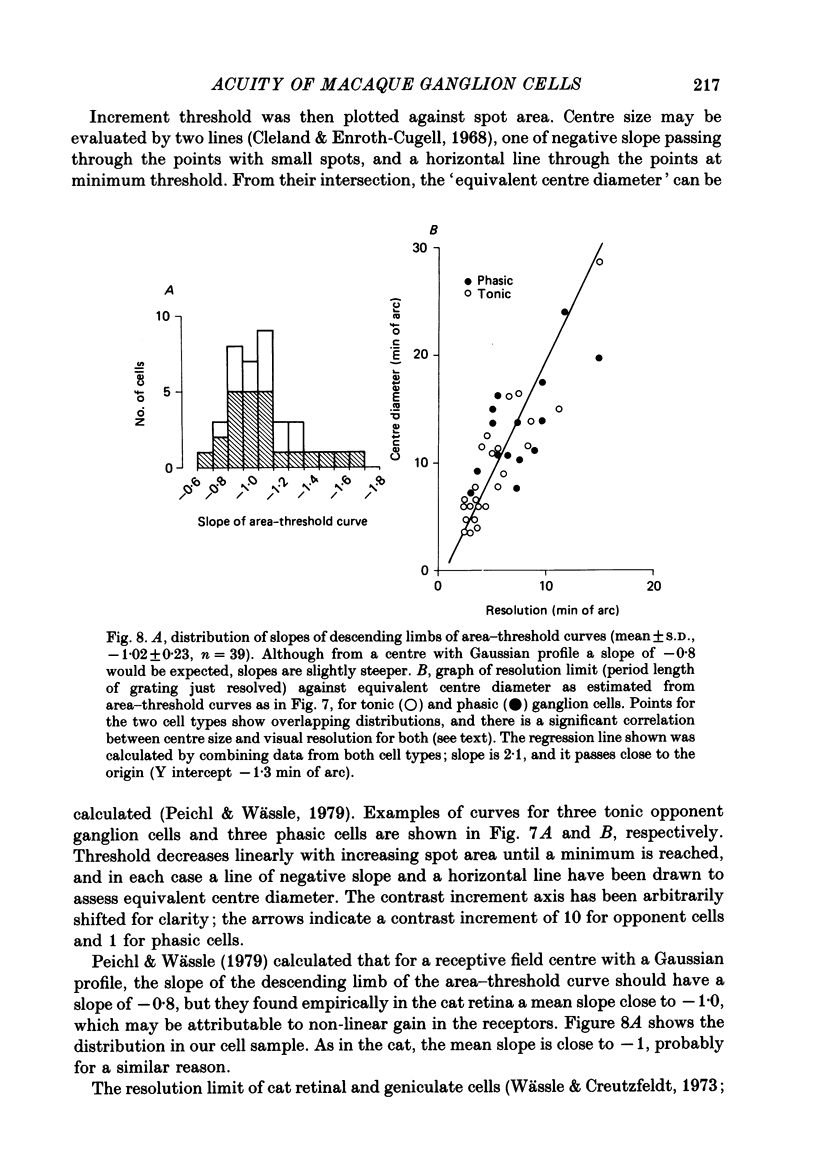
1. The visual resolving ability of different types of macaque retinal ganglion cells was estimated at different retinal eccentricities, by measuring the amplitude of modulated responses to black-white gratings of spatial frequencies near the resolution limit for each cell. 2. The resolving ability of tonic, spectrally opponent ganglion cells was usually similar to that of phasic, non-opponent ganglion cells at similar eccentricities, except that at eccentricities greater than 10 deg some tonic ganglion cells with remarkably high resolution (up to ca. 15 cycles/deg) were found. Our cell sample was limited within the central 2 deg of the visual field, however. 3. Only a small proportion of phasic ganglion cells showed an increase of mean firing level to gratings near the resolution limit. The maintained firing of tonic ganglion cells was higher than that of phasic ganglion cells. 4. With red-black or green-black gratings, the resolution of phasic ganglion cells was unaffected. For red or green on-centre ganglion cells, a marked deterioration of resolving ability occurred when the grating was of a colour to which a cell responded poorly (green-black gratings for red on-centre cells, and red-black gratings for green on-centre cells). A slight improvement in resolving ability occurred when the grating was of an excitatory colour. 5. For a sub-sample of cells, we compared resolution limit with centre size as determined from area-threshold curves. For both phasic and tonic ganglion cells, resolution limit (the period length just resolved) was about half the centre diameter, as is the case for cat ganglion cells. This implies that the centre sizes of phasic and tonic monkey ganglion cells are similar at most eccentricities. 6. We attempt to relate these results to primate retinal anatomy and visual resolution, determined behaviourally.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Vital-Durand F. Organization and post-natal development of the monkey's lateral geniculate nucleus. J Physiol. 1986 Nov;380:453–491. doi: 10.1113/jphysiol.1986.sp016297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Dartnall H. J., Mollon J. D. Microspectrophotometric demonstration of four classes of photoreceptor in an old world primate, Macaca fascicularis. J Physiol. 1980 Jan;298:131–143. doi: 10.1113/jphysiol.1980.sp013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Dartnall H. J. Visual pigments of rods and cones in a human retina. J Physiol. 1980 Jan;298:501–511. doi: 10.1113/jphysiol.1980.sp013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Gubisch R. W. Optical quality of the human eye. J Physiol. 1966 Oct;186(3):558–578. doi: 10.1113/jphysiol.1966.sp008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavonius C. R., Robbins D. O. Relationships between luminance and visual acuity in the rhesus monkey. J Physiol. 1973 Jul;232(2):239–246. doi: 10.1113/jphysiol.1973.sp010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman W. N., Jennings J. A. Objective measurements of the longitudinal chromatic aberration of the human eye. Vision Res. 1976;16(9):999–1005. doi: 10.1016/0042-6989(76)90232-7. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Harding T. H., Tulunay-Keesey U. Visual resolution and receptive field size: examination of two kinds of cat retinal ganglion cell. Science. 1979 Sep 7;205(4410):1015–1017. doi: 10.1126/science.472720. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Lee B. B., Elepfandt A. A quantitative study of chromatic organisation and receptive fields of cells in the lateral geniculate body of the rhesus monkey. Exp Brain Res. 1979 May 2;35(3):527–545. doi: 10.1007/BF00236770. [DOI] [PubMed] [Google Scholar]
- Crook J. M., Lee B. B., Tigwell D. A., Valberg A. Thresholds to chromatic spots of cells in the macaque geniculate nucleus as compared to detection sensitivity in man. J Physiol. 1987 Nov;392:193–211. doi: 10.1113/jphysiol.1987.sp016776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H. C., Polson M. C., Mead W. R., Hull E. M. Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res. 1974 Jan;14(1):53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks T. P., Lee B. B., Vidyasagar T. R. The responses of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol. 1983 Apr;337:183–200. doi: 10.1113/jphysiol.1983.sp014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. M. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982 Sep;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn B. J., Schnapf J. L., Baylor D. A. Spectral sensitivity of single cones in the retina of Macaca fascicularis. Nature. 1984 May 17;309(5965):264–266. doi: 10.1038/309264a0. [DOI] [PubMed] [Google Scholar]
- Peichl L., Wässle H. Size, scatter and coverage of ganglion cell receptive field centres in the cat retina. J Physiol. 1979 Jun;291:117–141. doi: 10.1113/jphysiol.1979.sp012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L., Wässle H. The structural correlate of the receptive field centre of alpha ganglion cells in the cat retina. J Physiol. 1983 Aug;341:309–324. doi: 10.1113/jphysiol.1983.sp014807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Cowey A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience. 1984 Aug;12(4):1125–1137. doi: 10.1016/0306-4522(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Cowey A. The ganglion cell and cone distributions in the monkey's retina: implications for central magnification factors. Vision Res. 1985;25(12):1795–1810. doi: 10.1016/0042-6989(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Regan D., Beverley K. I. Visual fields described by contrast sensitivity, by acuity, and by relative sensitivity to different orientations. Invest Ophthalmol Vis Sci. 1983 Jun;24(6):754–759. [PubMed] [Google Scholar]
- Rolls E. T., Cowey A. Topography of the retina and striate cortex and its relationship to visual acuity in rhesus monkeys and squirrel monkeys. Exp Brain Res. 1970;10(3):298–310. doi: 10.1007/BF00235053. [DOI] [PubMed] [Google Scholar]
- Rovamo J. Cortical magnification factor and contrast sensitivity to luminance-modulated chromatic gratings. Acta Physiol Scand. 1983 Dec;119(4):365–371. doi: 10.1111/j.1748-1716.1983.tb07351.x. [DOI] [PubMed] [Google Scholar]
- Snyder A. W., Miller W. H. Photoreceptor diameter and spacing for highest resolving power. J Opt Soc Am. 1977 May;67(5):696–698. doi: 10.1364/josa.67.000696. [DOI] [PubMed] [Google Scholar]
- Virsu V., Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Exp Brain Res. 1979;37(3):475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Wässle H., Boycott B. B., Illing R. B. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):177–195. doi: 10.1098/rspb.1981.0033. [DOI] [PubMed] [Google Scholar]
- Wässle H., Creutzfeldt O. D. Spatial resolution in visual system: a theoretical and experimental study on single units in the cat's lateral geniculate body. J Neurophysiol. 1973 Jan;36(1):13–27. doi: 10.1152/jn.1973.36.1.13. [DOI] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. Morphology and topography of on- and off-alpha cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):157–175. doi: 10.1098/rspb.1981.0032. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978 Nov;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]


