Abstract
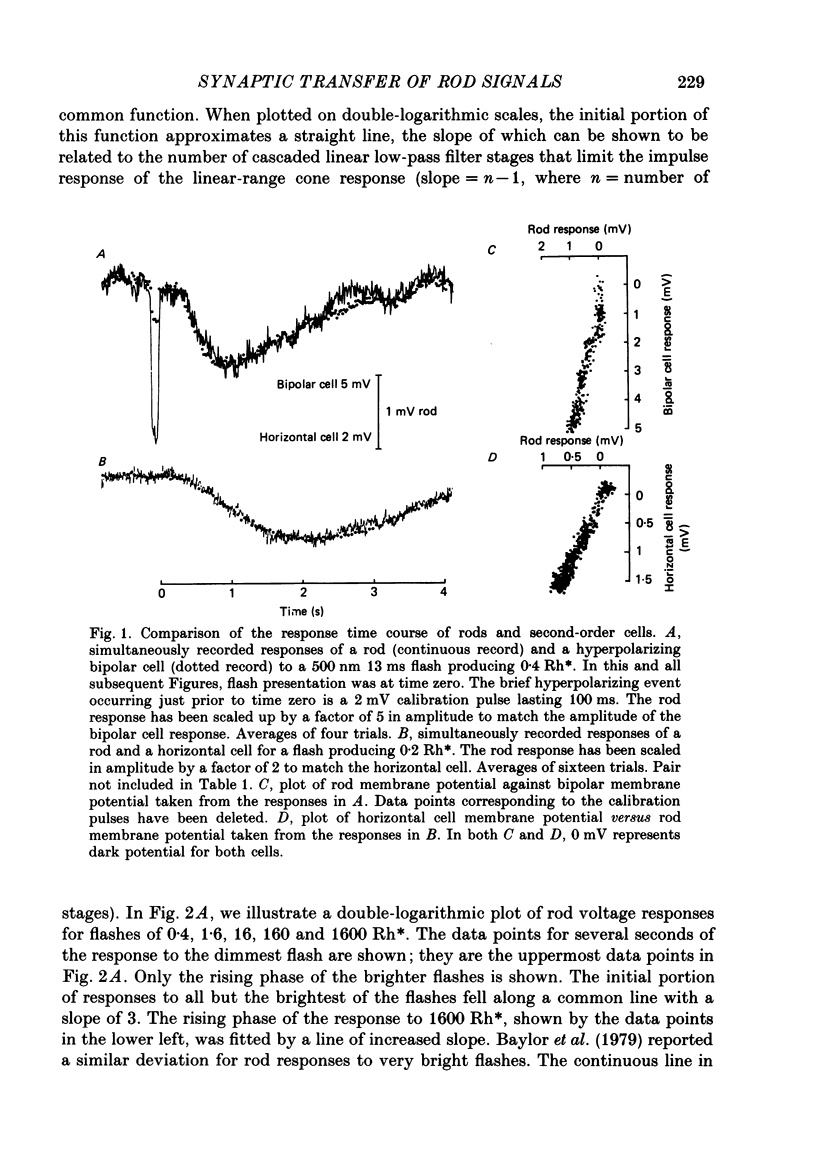
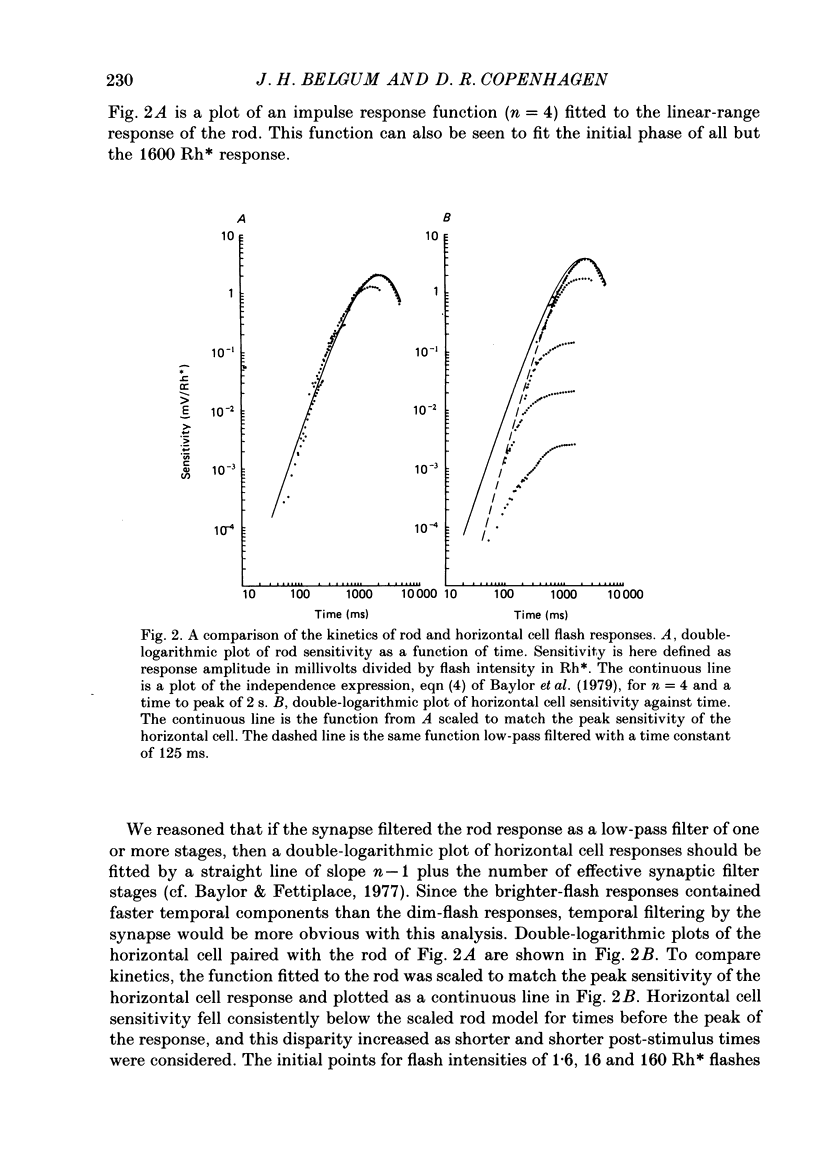
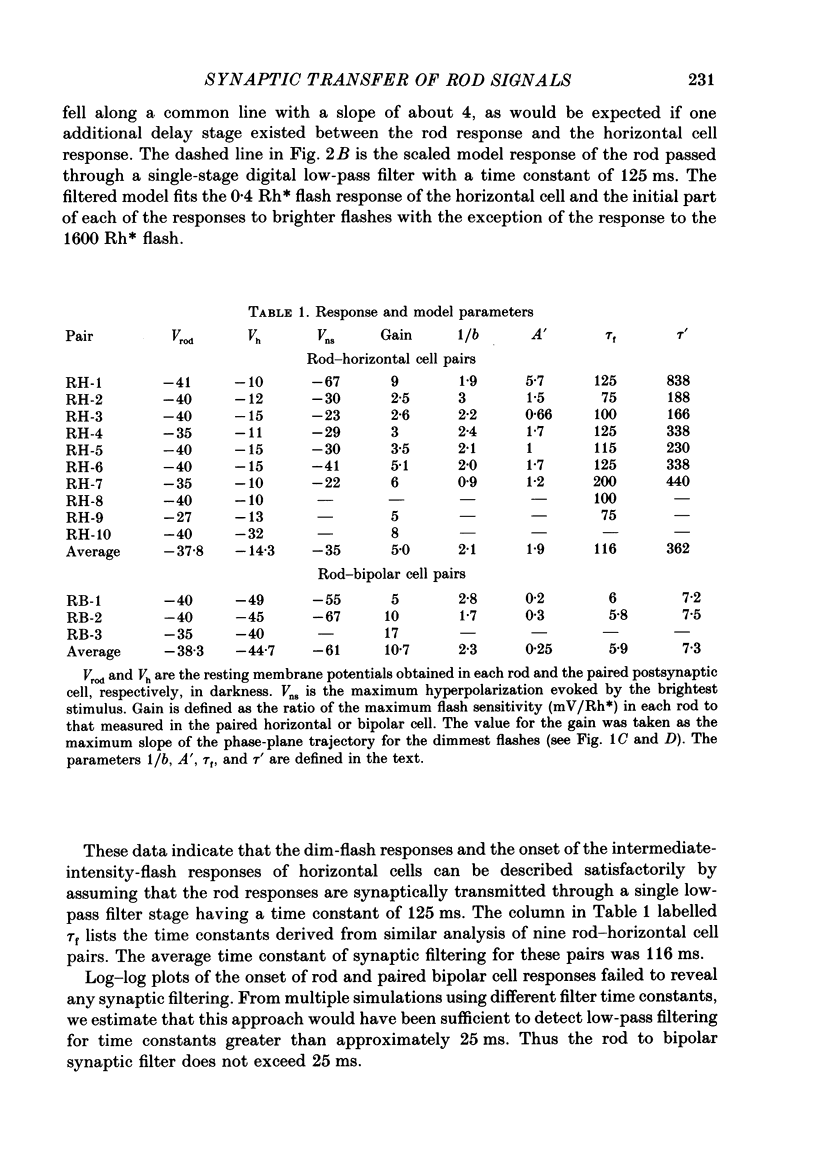
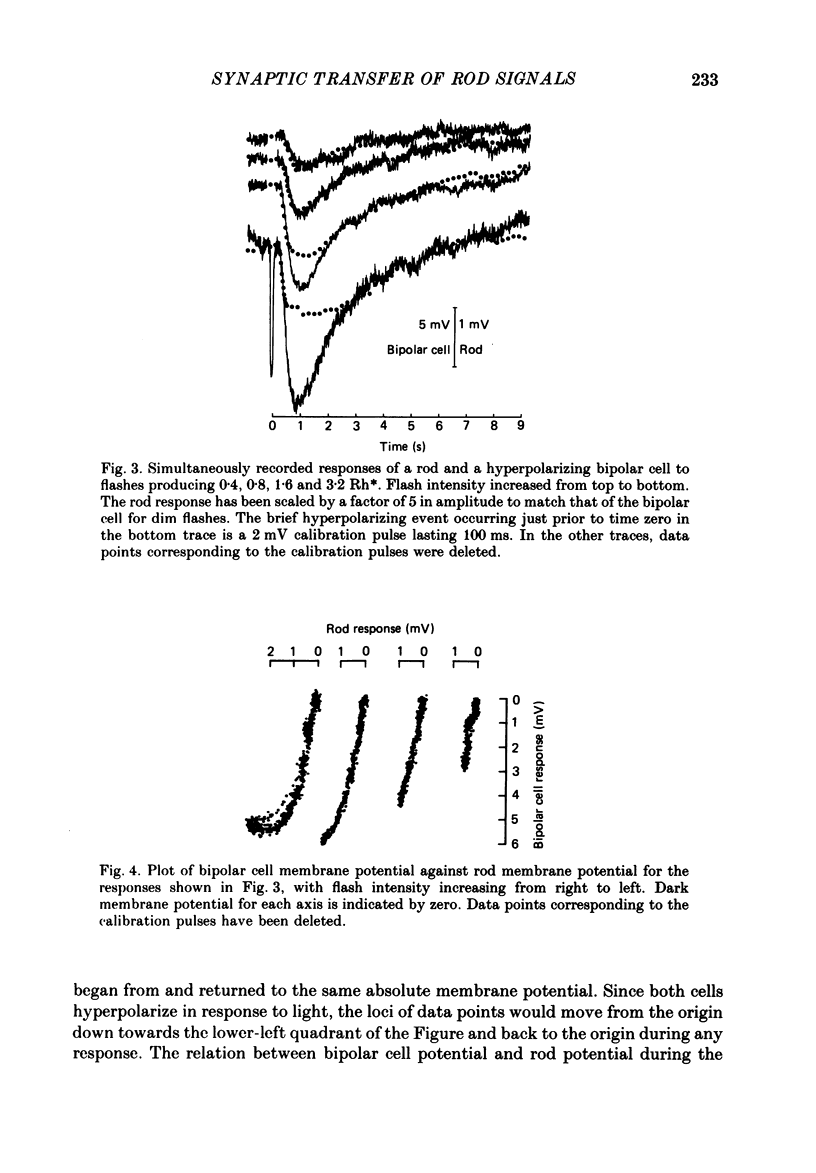
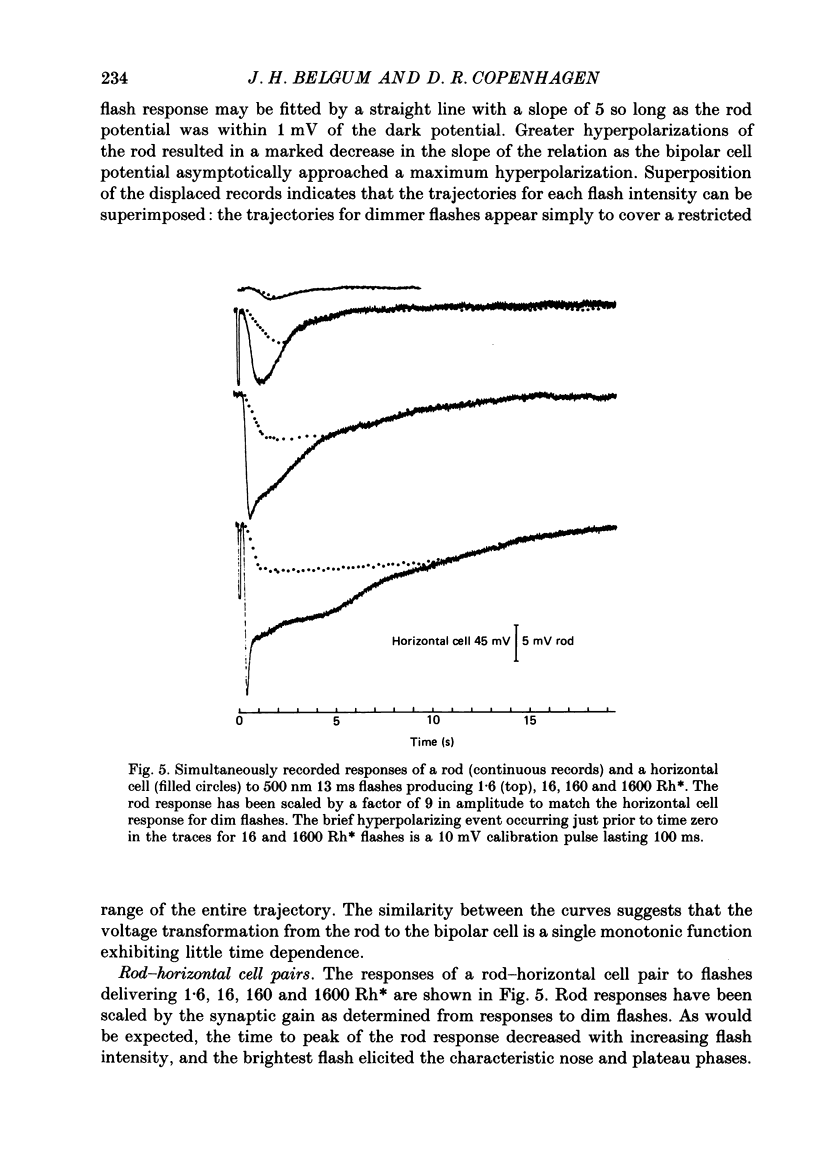
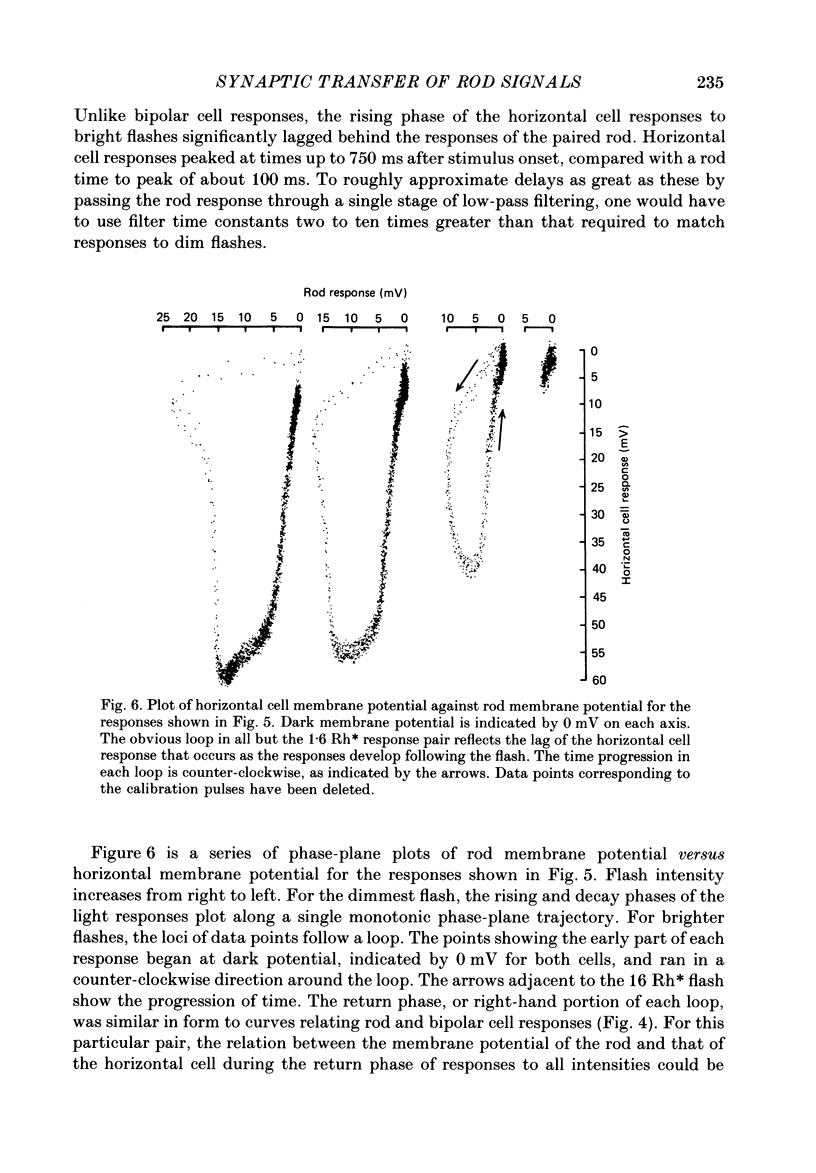
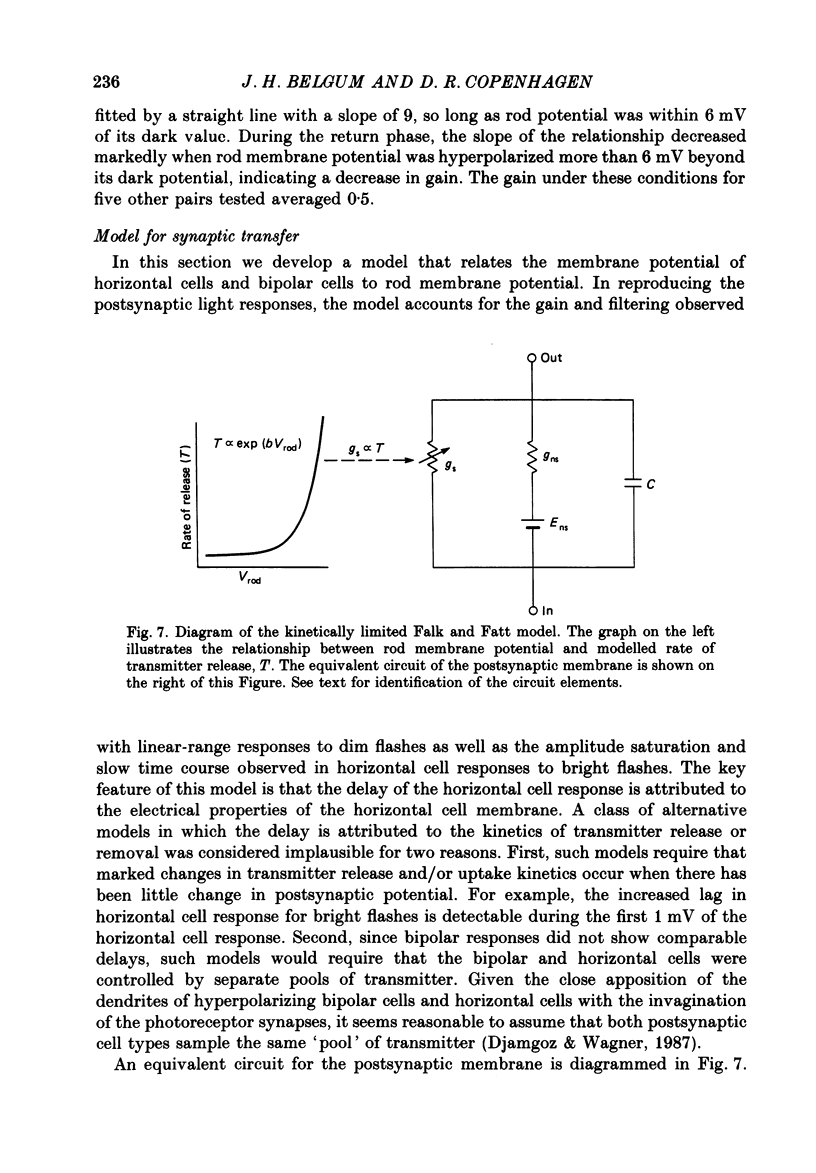
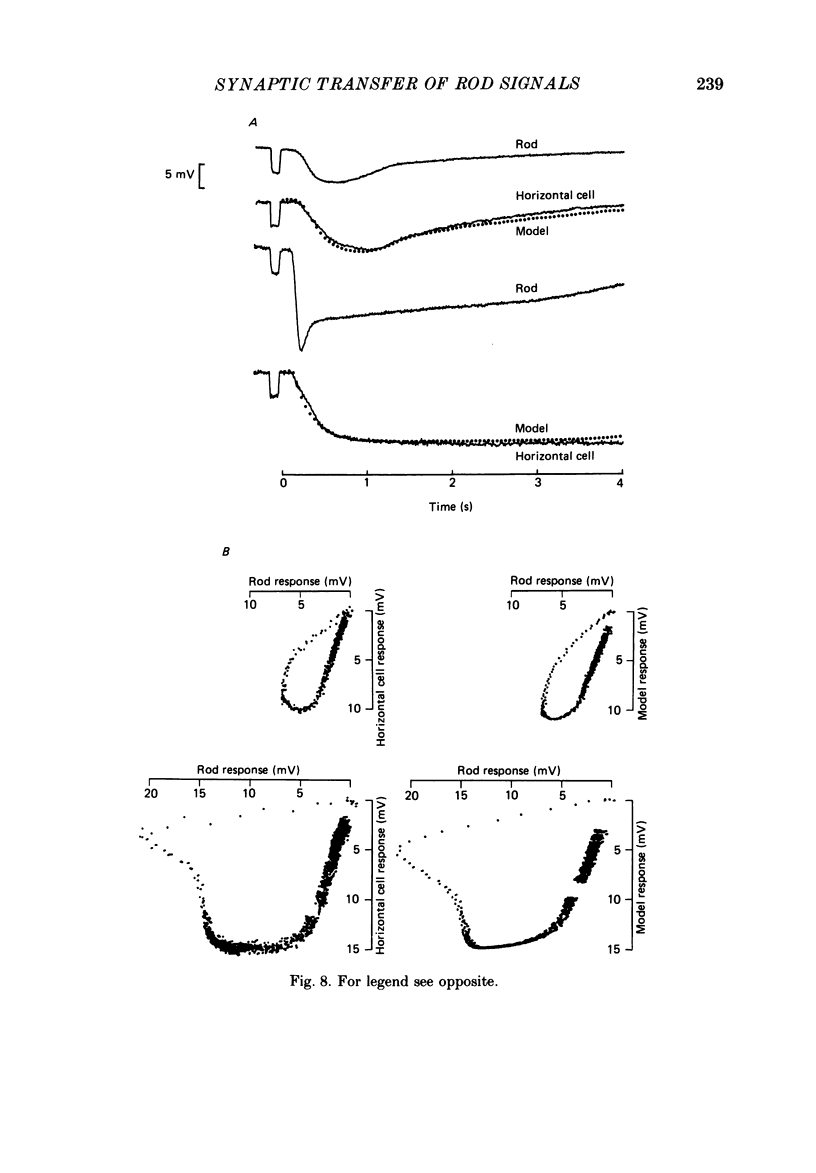
1. Simultaneous intracellular recordings of responses to light flashes were obtained from rod-horizontal cell and rod-hyperpolarizing bipolar cell pairs in isolated retinae of the toad. The gain and temporal filtering of synaptic transfer were characterized throughout the rods' range of light responses. 2. Paired rod-horizontal cell and rod-bipolar cell responses to dim flashes (less than 0.4 Rh*, where Rh* denotes effective photoisomerizations per rod per flash) exhibited nearly the same time course. Analysis of the onset of the horizontal cell responses revealed a temporal lag equivalent to a single stage of low-pass filtering (tau f = 75-200 ms). No filtering was discerned in the transfer of dim-flash responses from rods to bipolars. On average, horizontal cells were five times as sensitive (mV/Rh*) and hyperpolarizing bipolar cells 10.7 times as sensitive as their paired rods. 3. For brighter flashes, up to 1600 Rh*, the rising and return phases of bipolar responses appeared to be simple scaled versions of the rod responses. The scaling factor was equal to the ratio of flash sensitivities for dim flashes. Rod responses greater than about 2 mV produced a saturation of the bipolar cell response. 4. The return phases of the horizontal cell responses were kinetically similar, scaled versions of the rod responses for rod potentials less than about 5 mV. However, the rising phases lagged significantly behind those of the rod. The effective time constant of the lag increased proportionally with flash intensity. For the brighter flashes, the horizontal cell response peaked as much as a second after the rod response. 5. The linear scaling, minimal temporal filtering and saturation of the bipolar cell responses were satisfactorily reproduced by a model of synaptic transfer that assumed that the rate of transmitter release followed the rod voltage exponentially and that the postsynaptic conductance followed Michaelis-Menten saturation (Falk & Fatt, 1972). 6. The progressively longer lag in the horizontal cell responses to brighter flashes was satisfactorily simulated by a kinetically limited Falk and Fatt model which postulated that the effective electrical time constant of the horizontal cell membrane strongly depended on synaptic or voltage-modulated conductances. 7. Satisfactory model simulations of all postsynaptic responses required that an e-fold change in the release rate of transmitter from the rod be obtained with a 2 mV change in the rod potential.
Full text
PDF











Selected References
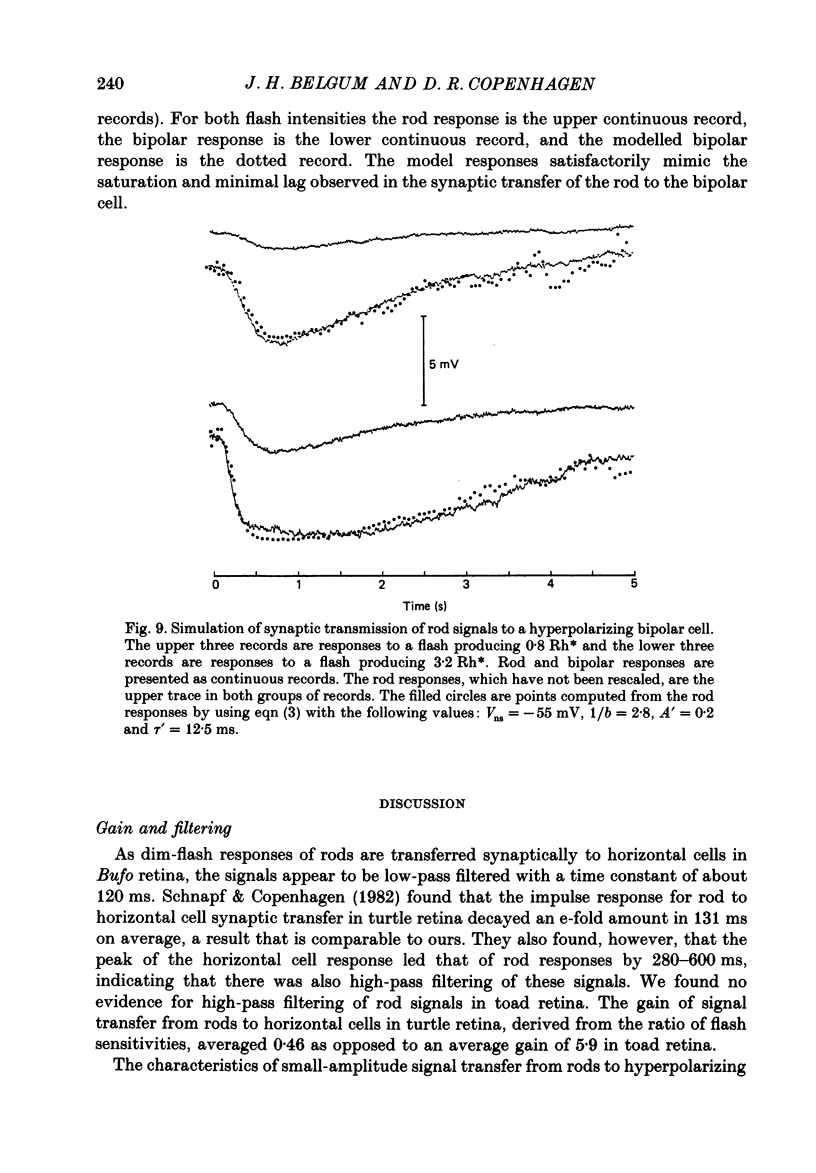
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Copenhagen D. R. An analysis of transmission from cones to hyperpolarizing bipolar cells in the retina of the turtle. J Physiol. 1983 Jul;340:569–597. doi: 10.1113/jphysiol.1983.sp014781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol. 1980 Mar;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Borges S., Wu S. M., Wilson M. Signal clipping by the rod output synapse. Nature. 1987 Aug 6;328(6130):522–524. doi: 10.1038/328522a0. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Kinetics of synaptic transfer from receptors to ganglion cells in turtle retina. J Physiol. 1977 Oct;271(2):425–448. doi: 10.1113/jphysiol.1977.sp012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov J. A. The response to electric stimulation of horizontal cells in the carp retina. Vision Res. 1968 Jul;8(7):817–822. doi: 10.1016/0042-6989(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Donner K., Reuter T. Ganglion cell performance at absolute threshold in toad retina: effects of dark events in rods. J Physiol. 1987 Dec;393:667–680. doi: 10.1113/jphysiol.1987.sp016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol. 1980 Sep;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973 Mar 9;242(5393):101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N. The effects of tetraethylammonium and cobalt ions on responses to extrinsic current in toad rods. J Physiol. 1980 Jun;303:515–533. doi: 10.1113/jphysiol.1980.sp013301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Synaptic transmission from photoreceptors to bipolar and horizontal cells in the carp retina. Cold Spring Harb Symp Quant Biol. 1976;40:537–546. doi: 10.1101/sqb.1976.040.01.050. [DOI] [PubMed] [Google Scholar]
- Leeper H. F., Copenhagen D. R. Mixed rod-cone responses in horizontal cells of snapping turtle retina. Vision Res. 1979;19(4):407–412. doi: 10.1016/0042-6989(79)90105-6. [DOI] [PubMed] [Google Scholar]
- MAURO A. Anomalous impedance, a phenomenological property of time-variant resistance. An analytic review. Biophys J. 1961 Mar;1:353–372. doi: 10.1016/s0006-3495(61)86894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S., Copenhagen D. R. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987 Jan 1;325(6099):56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Ripps H., Shakib M., MacDonald E. D. Peroxidase uptake by photoreceptor terminals of the skate retina. J Cell Biol. 1976 Jul;70(1):86–96. doi: 10.1083/jcb.70.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S. M., Holtzman E., Hood D. C. Uptake of horseradish peroxidase by frog photoreceptor synapses in the dark and the light. Nature. 1974 May 17;249(454):261–263. doi: 10.1038/249261a0. [DOI] [PubMed] [Google Scholar]
- Schnapf J. L., Copenhagen D. R. Differences in the kinetics of rod and cone synaptic transmission. Nature. 1982 Apr 29;296(5860):862–864. doi: 10.1038/296862a0. [DOI] [PubMed] [Google Scholar]
- Shingai R., Christensen B. N. Excitable properties and voltage-sensitive ion conductances of horizontal cells isolated from catfish (Ictalurus punctatus) retina. J Neurophysiol. 1986 Jul;56(1):32–49. doi: 10.1152/jn.1986.56.1.32. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Lamb T. D., Hodgkin A. L. Spontaneous voltage fluctuations in retinal cones and bipolar cells. Nature. 1975 Aug 21;256(5519):661–662. doi: 10.1038/256661a0. [DOI] [PubMed] [Google Scholar]
- Skrzypek J. Electrical coupling between horizontal cell bodies in the tiger salamander retina. Vision Res. 1984;24(7):701–711. doi: 10.1016/0042-6989(84)90211-6. [DOI] [PubMed] [Google Scholar]
- Thibos L. N., Werblin F. S. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol. 1978 May;278:79–99. doi: 10.1113/jphysiol.1978.sp012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Owen W. G. High-pass filtering of small signals by the rod network in the retina of the toad, Bufo marinus. Biophys J. 1983 Mar;41(3):305–324. doi: 10.1016/S0006-3495(83)84443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wojtowicz J. M., Atwood H. L. Presynaptic membrane potential and transmitter release at the crayfish neuromuscular junction. J Neurophysiol. 1984 Jul;52(1):99–113. doi: 10.1152/jn.1984.52.1.99. [DOI] [PubMed] [Google Scholar]
- Wu S. M. Synaptic transmission from rods to bipolar cells in the tiger salamander retina. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3944–3947. doi: 10.1073/pnas.82.11.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]


