Abstract
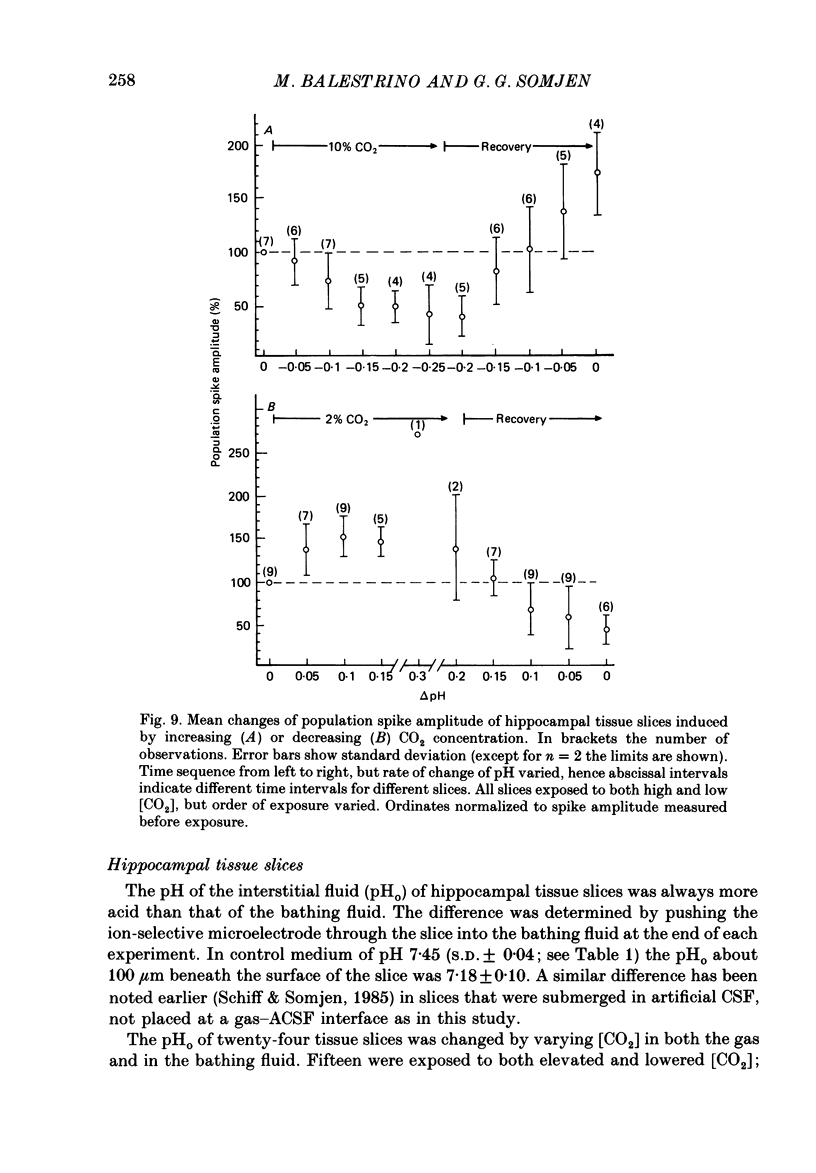
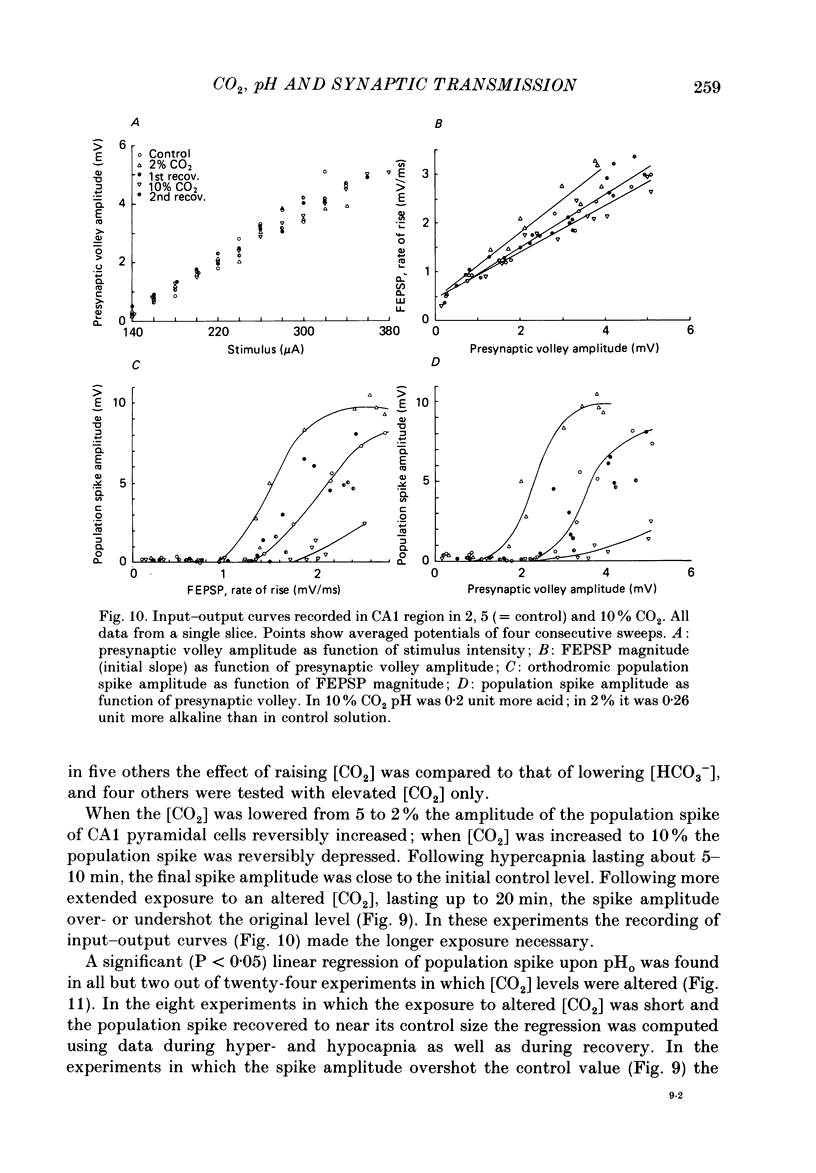
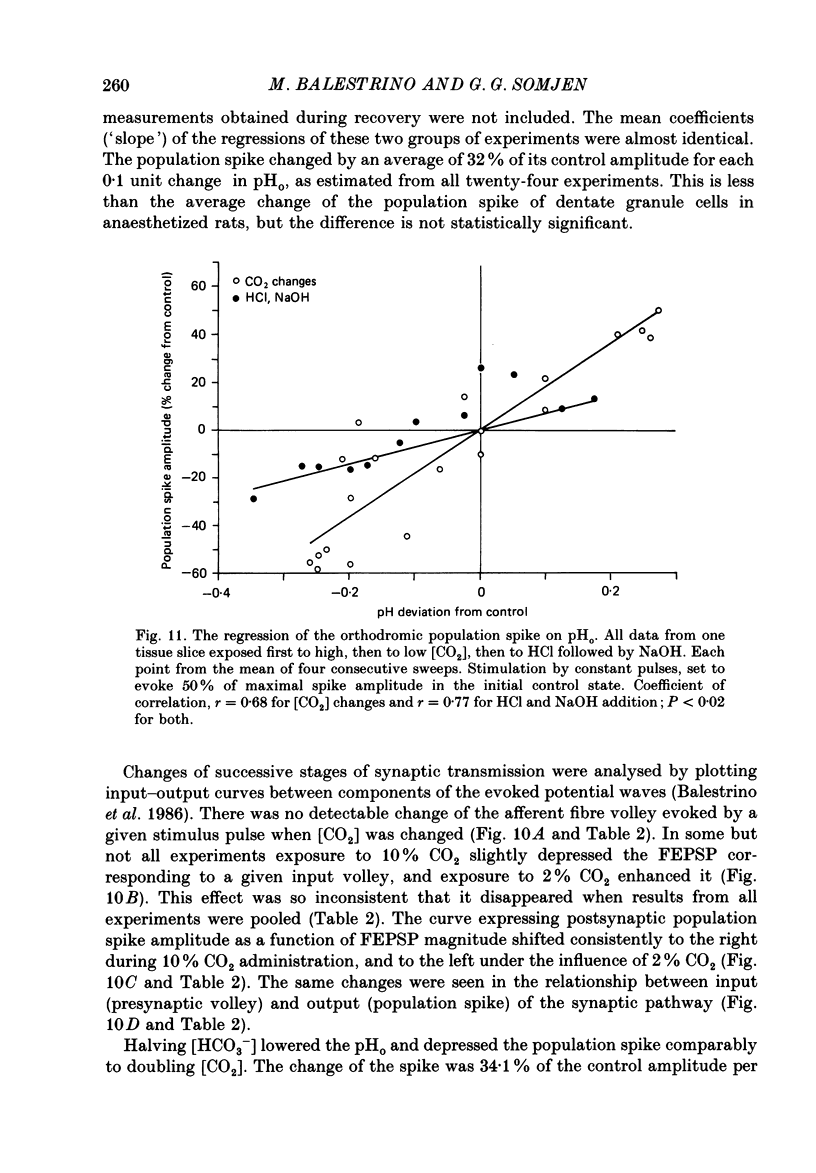
1. Interstitial pH (pHo) was measured with ion-selective microelectrodes in the fascia dentata of rats anaesthetized with urethane, while CO2 levels were controlled by varying pulmonary ventilation and CO2 content of inspired air. In the CA1 sector of hippocampal tissue slices in vitro pHo was similarly measured and altered by varying CO2 in the gas phase, or by adding HCl or NaOH to the artificial cerebrospinal fluid (ACSF) of the bath, or by changing the concentration of HCO3-. 2. Orthodromically evoked compound action potentials ('population spikes') were depressed in hypercapnia and increased in hypocapnia. In the fascia dentata of intact brains the population spike of the granule cells varied on average by more than 40% of control amplitude for each 0.1 change of pHo. In the CA1 zone of tissue slices in vitro, the change of population spike amplitude was approximately 30% per pH change of 0.1 caused by altered CO2 or HCO3- concentration, but only about 15% per pH change of 0.1 when HCl or NaOH were administered. 3. In anaesthetized rats the focal synaptic potential (FEPSP) evoked by a given stimulus intensity was weakly influenced by varying [CO2]; in tissue slices weak effects on FEPSP were inconsistent. In hippocampus both in situ and in vitro the population spike triggered by a given magnitude of FEPSP increased in hypocapnia and decreased in hypercapnia. This suggests that the main effect of CO2 is on the electric excitability of postsynaptic cells, with minor or no effect on transmitter release and on the interaction of the transmitter with its receptors. 4. Hypercapnia of anaesthetized rats was usually associated with a slight increase of [K+]o in the fascia dentata. Tissue [Ca2+]o changed little and not consistently. Neither of these two ions, nor concomitant changes of blood pressure or tissue partial pressure of oxygen, (Pt, O2), could account for the effects of pH on neuronal excitability. 5. The results show that increasing the extracellular concentration of H+ ions has a moderately depressant effect on the firing threshold of hippocampal neurones. The more powerful effects of elevated [CO2] and of lowered [HCO3-] may probably be explained by a direct effect on the neuronal membrane. The brain, by regulating breathing, controls its own excitability.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken P. G. Kainic acid and penicillin: differential effects on excitatory and inhibitory interactions in the CA1 region of the hippocampal slice. Brain Res. 1985 Jan 28;325(1-2):261–269. doi: 10.1016/0006-8993(85)90322-1. [DOI] [PubMed] [Google Scholar]
- BROWN E. B., Jr Physiological effects of hyperventilation. Physiol Rev. 1953 Oct;33(4):445–471. doi: 10.1152/physrev.1953.33.4.445. [DOI] [PubMed] [Google Scholar]
- Balestrino M., Aitken P. G., Somjen G. G. The effects of moderate changes of extracellular K+ and Ca2+ on synaptic and neural function in the CA1 region of the hippocampal slice. Brain Res. 1986 Jul 9;377(2):229–239. doi: 10.1016/0006-8993(86)90863-2. [DOI] [PubMed] [Google Scholar]
- CLARK L. C., Jr, MISRAHY G., FOX R. P. Chronically implanted polarographic electrodes. J Appl Physiol. 1958 Jul;13(1):85–91. doi: 10.1152/jappl.1958.13.1.85. [DOI] [PubMed] [Google Scholar]
- Cameron I. R., Davson H., Segal M. B. The effect of hypercapnia on the blood-brain barrier to sucrose in the rabbit. Yale J Biol Med. 1969 Dec;42(3-4):241–247. [PMC free article] [PubMed] [Google Scholar]
- Caspers H., Speckmann E. J. Cerebral pO 2 , pCO 2 and pH: changes during convulsive activity and their significance for spontaneous arrest of seizures. Epilepsia. 1972 Sep;13(5):699–725. doi: 10.1111/j.1528-1157.1972.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol. 1968 Mar;31(2):142–165. doi: 10.1152/jn.1968.31.2.142. [DOI] [PubMed] [Google Scholar]
- Cragg P., Patterson L., Purves M. J. The pH of brain extracellular fluid in the cat. J Physiol. 1977 Oct;272(1):137–166. doi: 10.1113/jphysiol.1977.sp012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DETTBARN W. D., STAMPFLI R. Die Wirkung von 2, 4-Dinitrophenol auf das Membranpotential der markhaltigen Nervenfaser. Helv Physiol Pharmacol Acta. 1957;15(1):25–37. [PubMed] [Google Scholar]
- De Weer P. Intracellular pH transients induced by Co2 or NH3. Respir Physiol. 1978 Apr;33(1):41–50. doi: 10.1016/0034-5687(78)90082-8. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Somjen G. Calcium dependence of synaptic transmission in the hippocampal slice. Brain Res. 1981 Feb 23;207(1):218–222. doi: 10.1016/0006-8993(81)90697-1. [DOI] [PubMed] [Google Scholar]
- GOTOH F., MEYER J. S., TAKAGI Y. CEREBRAL EFFECTS OF HYPERVENTILATION IN MAN. Arch Neurol. 1965 Apr;12:410–423. doi: 10.1001/archneur.1965.00460280080008. [DOI] [PubMed] [Google Scholar]
- Gillette R. Intracellular alkalinization potentiates slow inward current and prolonged bursting in a molluscan neuron. J Neurophysiol. 1983 Feb;49(2):509–515. doi: 10.1152/jn.1983.49.2.509. [DOI] [PubMed] [Google Scholar]
- KIRSTEIN L. Early effects of oxygen lack and carbon dioxide excess on spinal reflexes. Acta Physiol Scand Suppl. 1951;23(80):1–54. [PubMed] [Google Scholar]
- KRNJEVIC K., RANDIC M., SIESJOE B. K. CORTICAL CO2 TENSION AND NEURONAL EXCITABILITY. J Physiol. 1965 Jan;176:105–122. doi: 10.1113/jphysiol.1965.sp007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S. S., Schmidt C. F. THE EFFECTS OF ALTERED ARTERIAL TENSIONS OF CARBON DIOXIDE AND OXYGEN ON CEREBRAL BLOOD FLOW AND CEREBRAL OXYGEN CONSUMPTION OF NORMAL YOUNG MEN. J Clin Invest. 1948 Jul;27(4):484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisman N. R., Sick T. J., LaManna J. C., Rosenthal M. Local tissue oxygen tension-cytochrome a,a3 redox relationships in rat cerebral cortex in vivo. Brain Res. 1981 Aug 10;218(1-2):161–174. doi: 10.1016/0006-8993(81)91298-1. [DOI] [PubMed] [Google Scholar]
- Lothman E., Lamanna J., Cordingley G., Rosenthal M., Somjen G. Responses of electrical potential, potassium levels, and oxidative metabolic activity of the cerebral neocortex of cats. Brain Res. 1975 Apr 25;88(1):15–36. doi: 10.1016/0006-8993(75)90943-9. [DOI] [PubMed] [Google Scholar]
- MEYER J. S., GOTOH F. Metabolic and electroencephalographic effects of hyperventilation. Experimental studies of brain oxygen and carbon dioxide tension, pH, EEG and blood flow during hyperventilation. Arch Neurol. 1960 Nov;3:539–552. doi: 10.1001/archneur.1960.00450050059007. [DOI] [PubMed] [Google Scholar]
- Moody W., Jr Effects of intracellular H+ on the electrical properties of excitable cells. Annu Rev Neurosci. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Nattie E. E. Ionic mechanisms of cerebrospinal fluid acid-base regulation. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):3–12. doi: 10.1152/jappl.1983.54.1.3. [DOI] [PubMed] [Google Scholar]
- Papajewski W., Klee M. R., Wagner A. The action of raised CO2 pressure on the excitability of spinal motorneurones. Electroencephalogr Clin Neurophysiol. 1969 Dec;27(6):618–618. [PubMed] [Google Scholar]
- SUGIOKA K., DAVIS D. A. Hyperventilation with oxygen-a possible cause of cerebral hypoxia. Anesthesiology. 1960 Mar-Apr;21:135–143. doi: 10.1097/00000542-196003000-00001. [DOI] [PubMed] [Google Scholar]
- Schiff S. J., Somjen G. G. Overshoot of oxygen pressure in post-hypoxic brain tissue: a re-evaluation. Brain Res. 1985 Sep 30;344(1):150–153. doi: 10.1016/0006-8993(85)91200-4. [DOI] [PubMed] [Google Scholar]
- Schlue W. R., Thomas R. C. A dual mechanism for intracellular pH regulation by leech neurones. J Physiol. 1985 Jul;364:327–338. doi: 10.1113/jphysiol.1985.sp015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö B. K., von Hanwehr R., Nergelius G., Nevander G., Ingvar M. Extra- and intracellular pH in the brain during seizures and in the recovery period following the arrest of seizure activity. J Cereb Blood Flow Metab. 1985 Mar;5(1):47–57. doi: 10.1038/jcbfm.1985.7. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res. 1984 Oct 8;311(1):186–188. doi: 10.1016/0006-8993(84)91416-1. [DOI] [PubMed] [Google Scholar]
- Somjen G. G., Aitken P. G., Giacchino J. L., McNamara J. O. Sustained potential shifts and paroxysmal discharges in hippocampal formation. J Neurophysiol. 1985 Apr;53(4):1079–1097. doi: 10.1152/jn.1985.53.4.1079. [DOI] [PubMed] [Google Scholar]
- Somjen G. G., Allen B. W., Balestrino M., Aitken P. G. Pathophysiology of pH and Ca2+ in bloodstream and brain. Can J Physiol Pharmacol. 1987 May;65(5):1078–1085. doi: 10.1139/y87-169. [DOI] [PubMed] [Google Scholar]
- TSCHIRGI R. D., TAYLOR J. L. Slowly changing bioelectric potentials associated with the blood-brain barrier. Am J Physiol. 1958 Oct;195(1):7–22. doi: 10.1152/ajplegacy.1958.195.1.7. [DOI] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Jr, Brown A. M. Unified account of the variable effects of carbon dioxide on nerve cells. Science. 1970 Mar 13;167(3924):1502–1504. doi: 10.1126/science.167.3924.1502. [DOI] [PubMed] [Google Scholar]
- Yachnis A. T., Crawley J. N., Jensen R. T., McGrane M. M., Moody T. W. The antagonism of bombesin in the CNS by substance P analogues. Life Sci. 1984 Nov 5;35(19):1963–1969. doi: 10.1016/0024-3205(84)90477-6. [DOI] [PubMed] [Google Scholar]


