Abstract
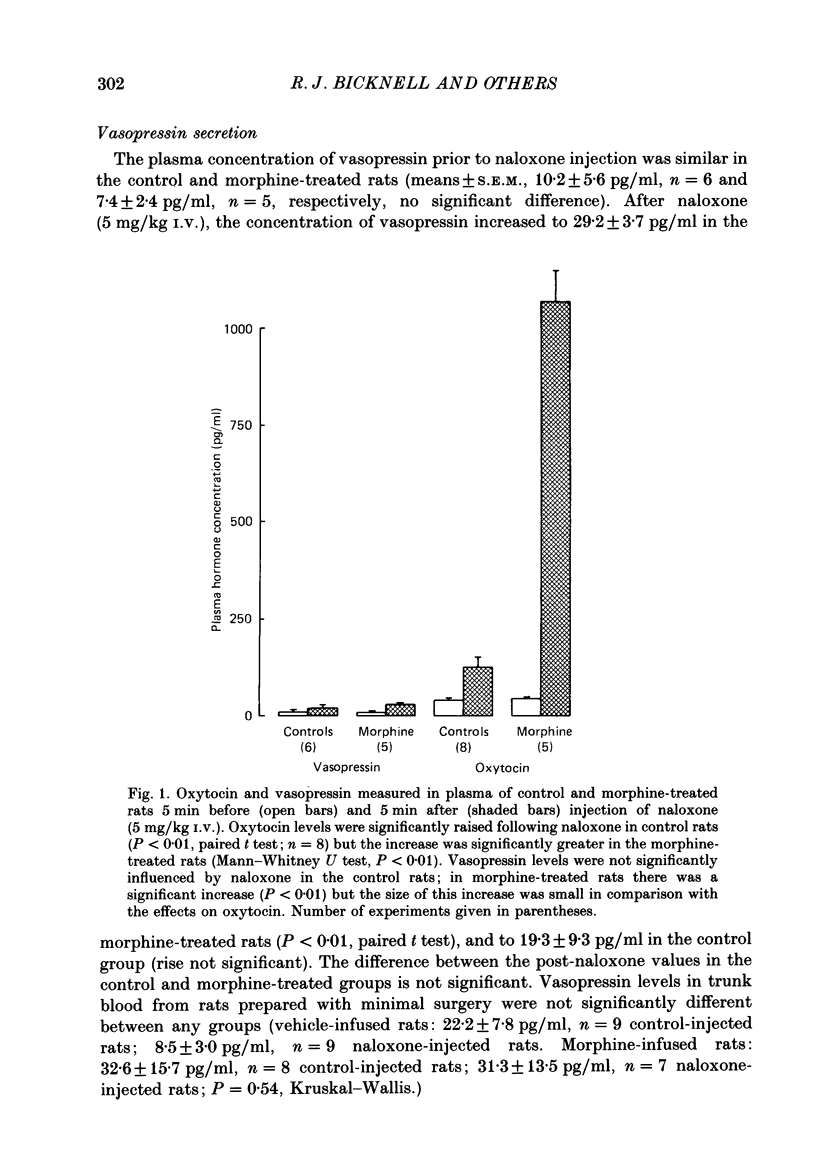
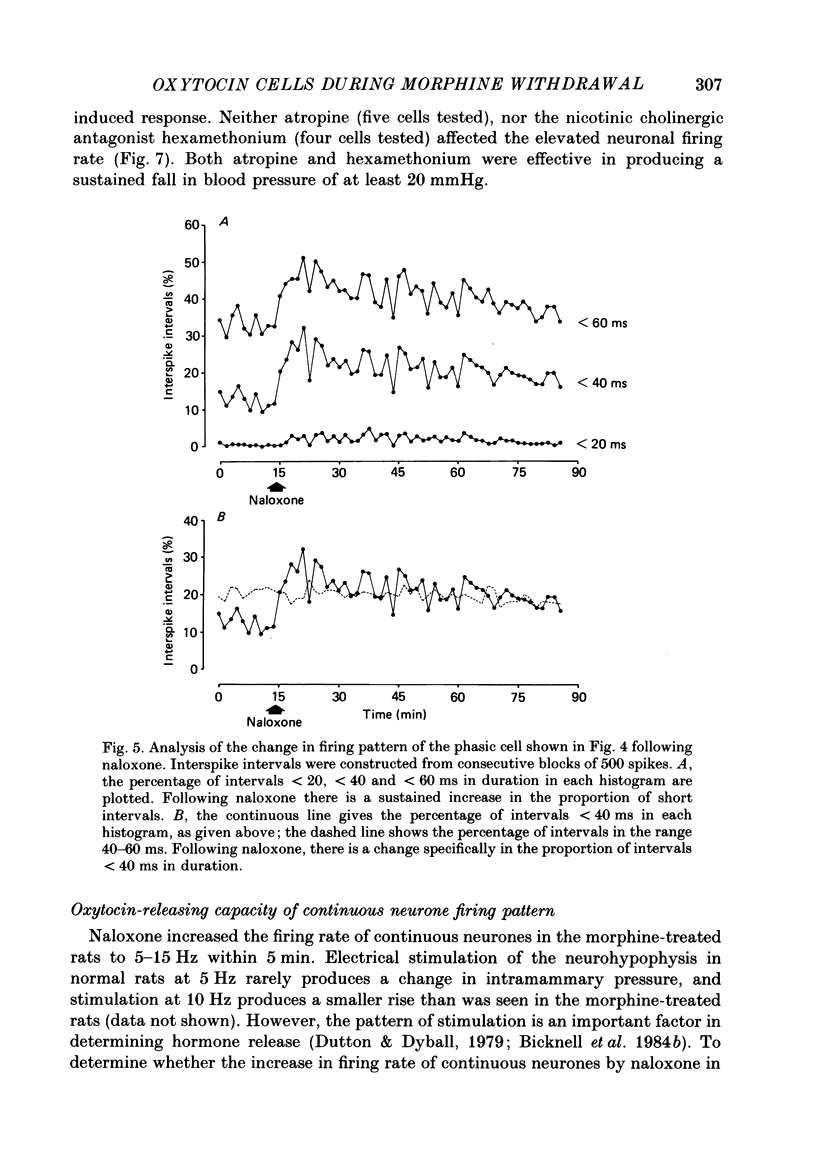
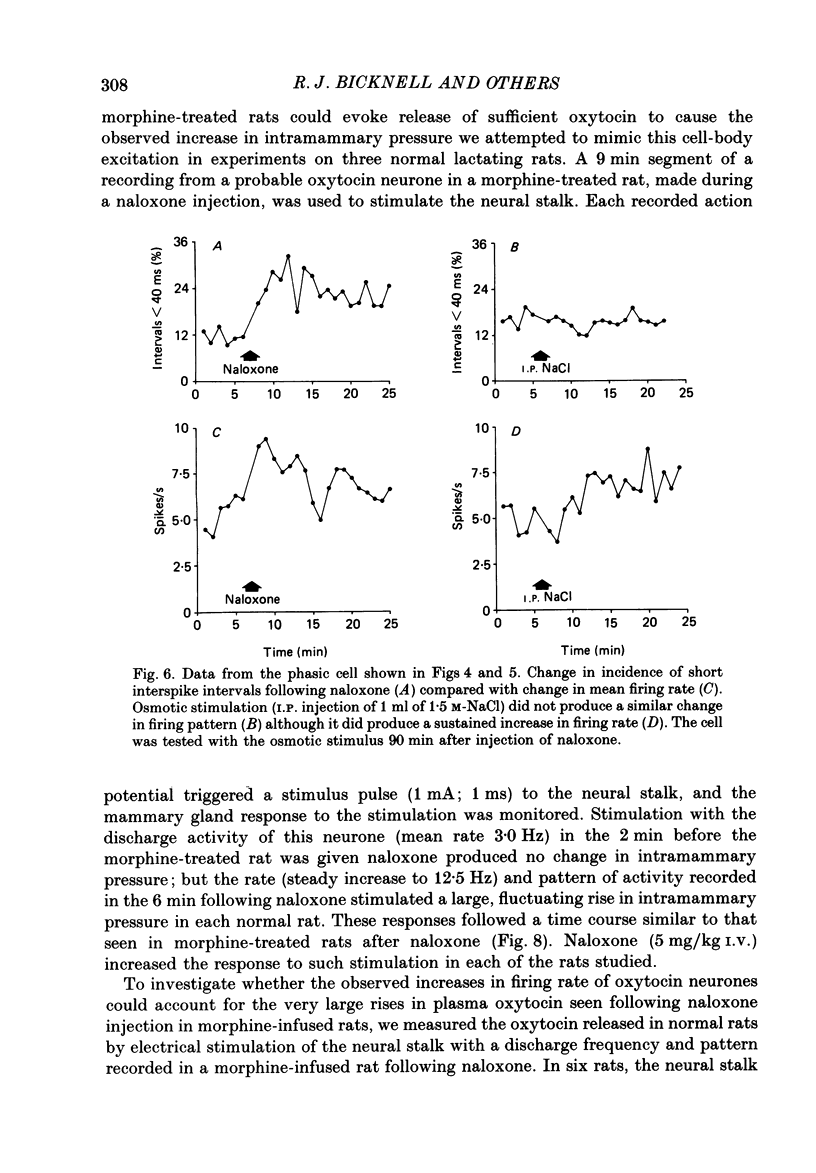
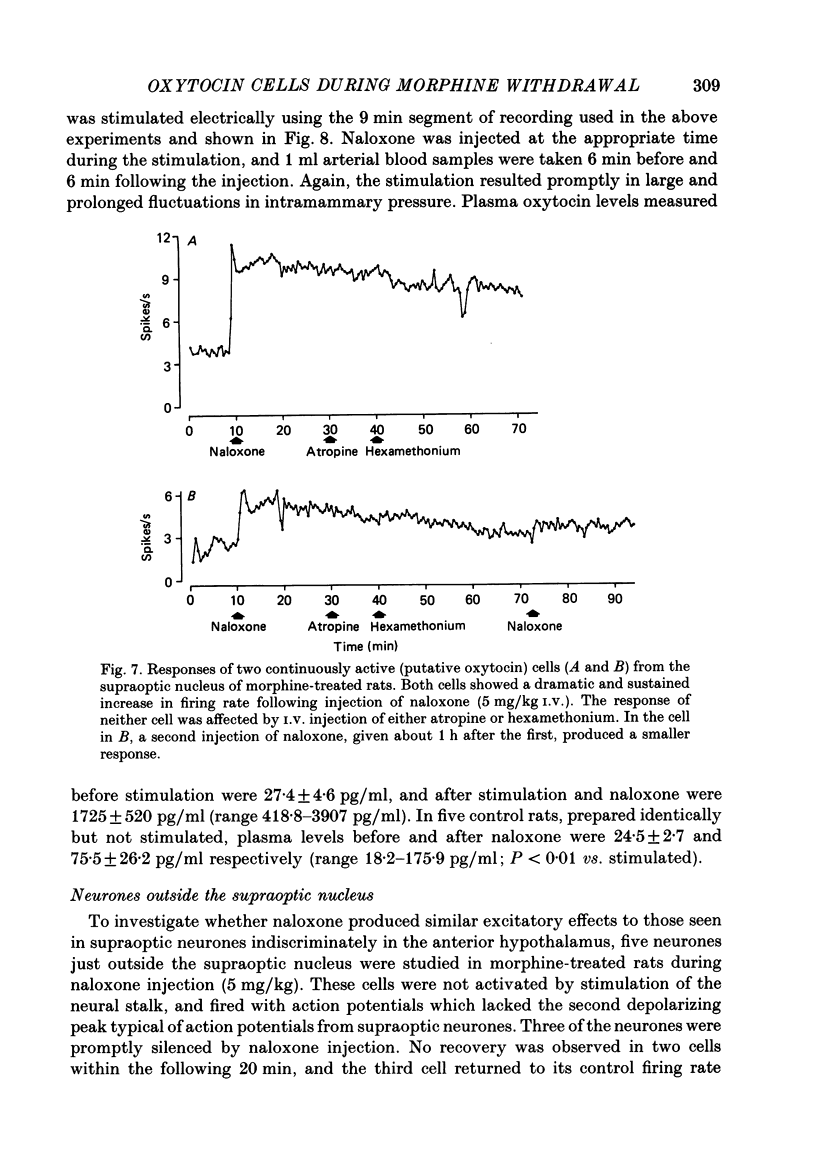
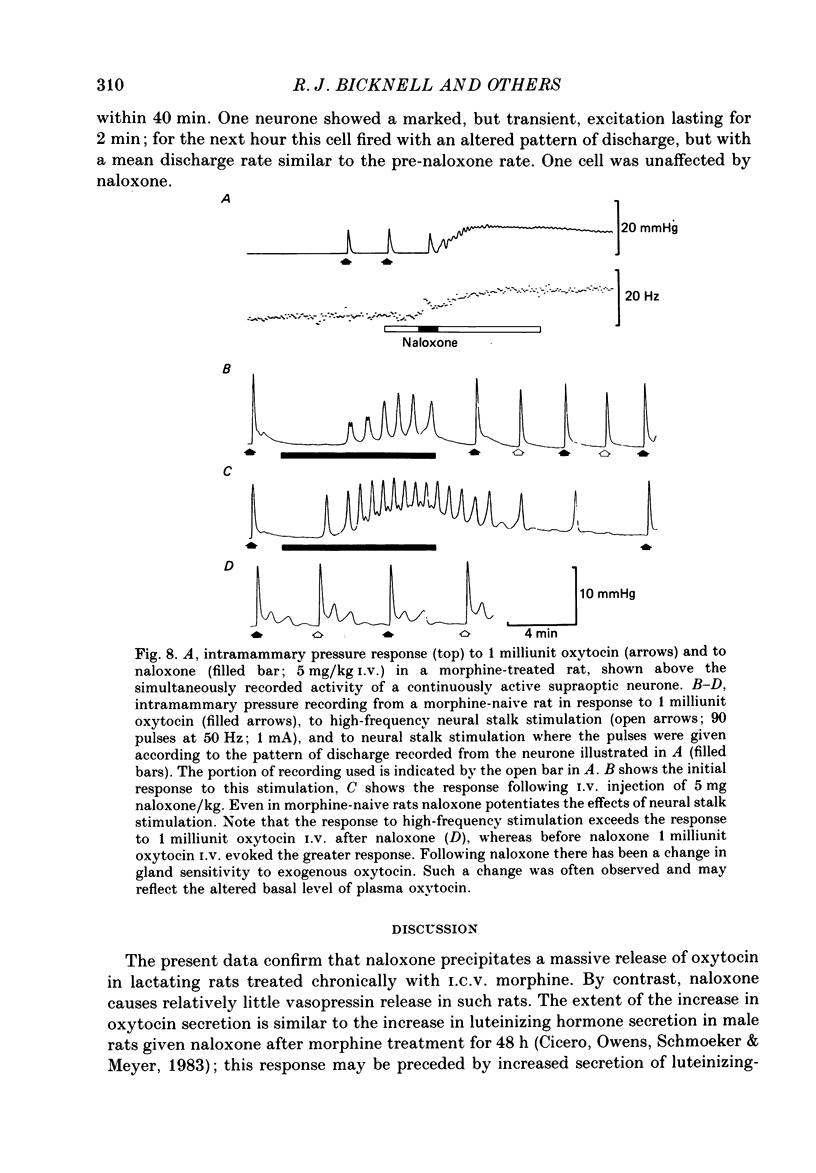
1. Lactating rats were implanted with a cannula in a lateral cerebral ventricle to deliver morphine (up to 50 micrograms/h) chronically from a subcutaneous osmotically driven mini-pump. After infusion of morphine for 5 days the rats were anaesthetized with urethane and prepared with ventral surgery for recording the electrical activity of single, antidromically identified neurones in the supraoptic nucleus. 2. A single I.V. injection of naloxone (5 mg/kg) in these rats provoked a long-lasting, large increase in intramammary pressure, but in control rats had negligible effects. Concentrations in plasma of oxytocin, measured by radioimmunoassay in samples of femoral arterial blood, rose from 44.7 +/- 2.5 to 1072.1 +/- 89.5 pg/ml (means +/- S.E.M.) 6 min after naloxone in the morphine-treated rats. In control rats, the concentration of oxytocin in plasma rose only from 42.1 +/- 2.9 to 125.1 +/- 28.2 pg/ml after naloxone. 3. Naloxone produced a transient increase in arterial blood pressure in morphine-treated but not control rats. Concentrations in plasma of vasopressin, measured by radioimmunoassay in samples of femoral arterial blood, rose in morphine-treated rats from 7.4 +/- 2.4 to 29.2 +/- 3.7 pg/ml after naloxone, but did not rise significantly in control rats. 4. Naloxone (1-5 mg/kg) produced a prompt and prolonged increase in the discharge rate of each of ten continuously active (putative oxytocin) cells recorded from ten morphine-treated rats. The discharge rate of the six cells tested at the highest dose (5 mg/kg) increased by an average of 6.3 Hz (360%) within 5 min, and the firing rate remained elevated for at least 30 min; the discharge rate of six continuously active supraoptic neurones recorded in control rats was not affected by naloxone. 5. The firing activity of five phasic (putative vasopressin) supraoptic neurones in morphine-treated rats was increased for at least 30 min by the injection of naloxone; these increases were the result of a raised intraburst firing rate with no change in burst duration or frequency. One phasic neurone was inhibited for 15 min, and one phasic neurone was unaffected. 6. The excitatory effects of naloxone on neurones in the supraoptic nucleus of morphine-treated rats were not explained by changes in blood pressure or osmolarity and did not depend on suckling or a cholinergic pathway. 7. The concentrations of oxytocin in plasma and the operation of the milk-ejection reflex were similar in the controls and morphine-treated rats, prior to naloxone. These findings indicate tolerance to initial inhibitory effects of morphine on oxytocin secretion.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





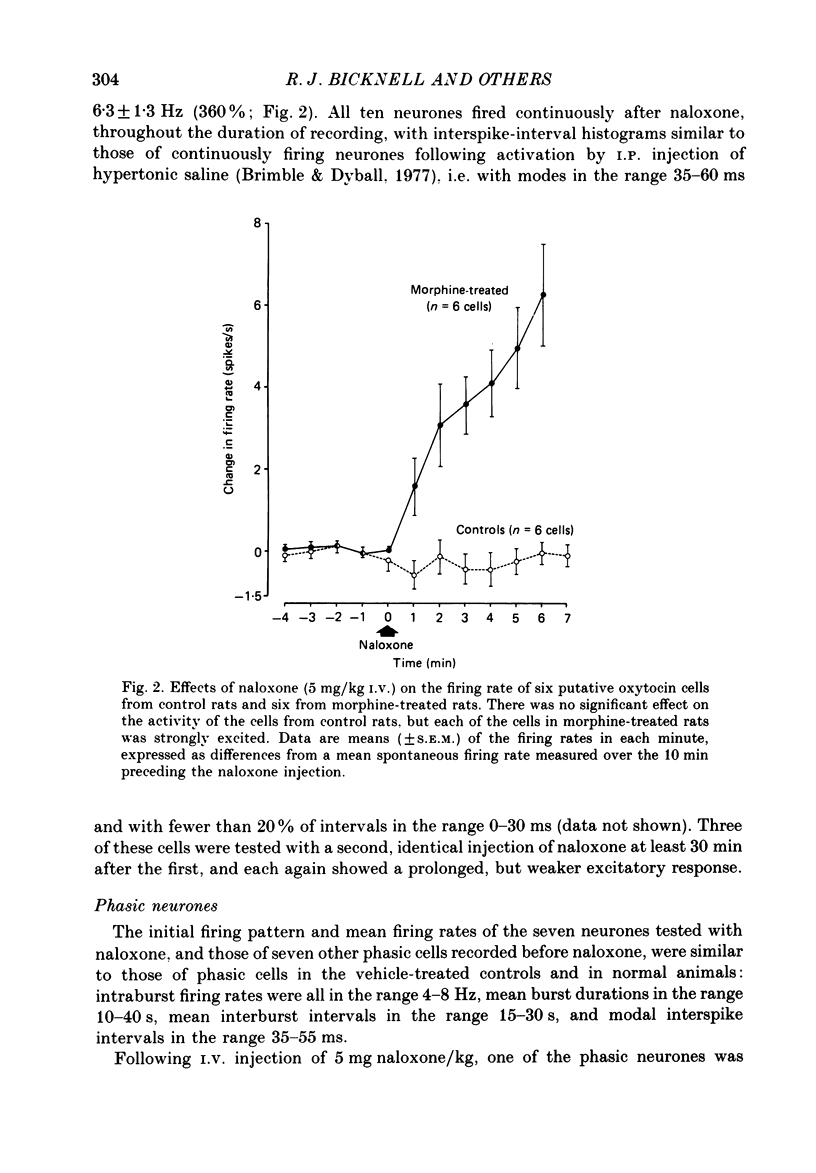
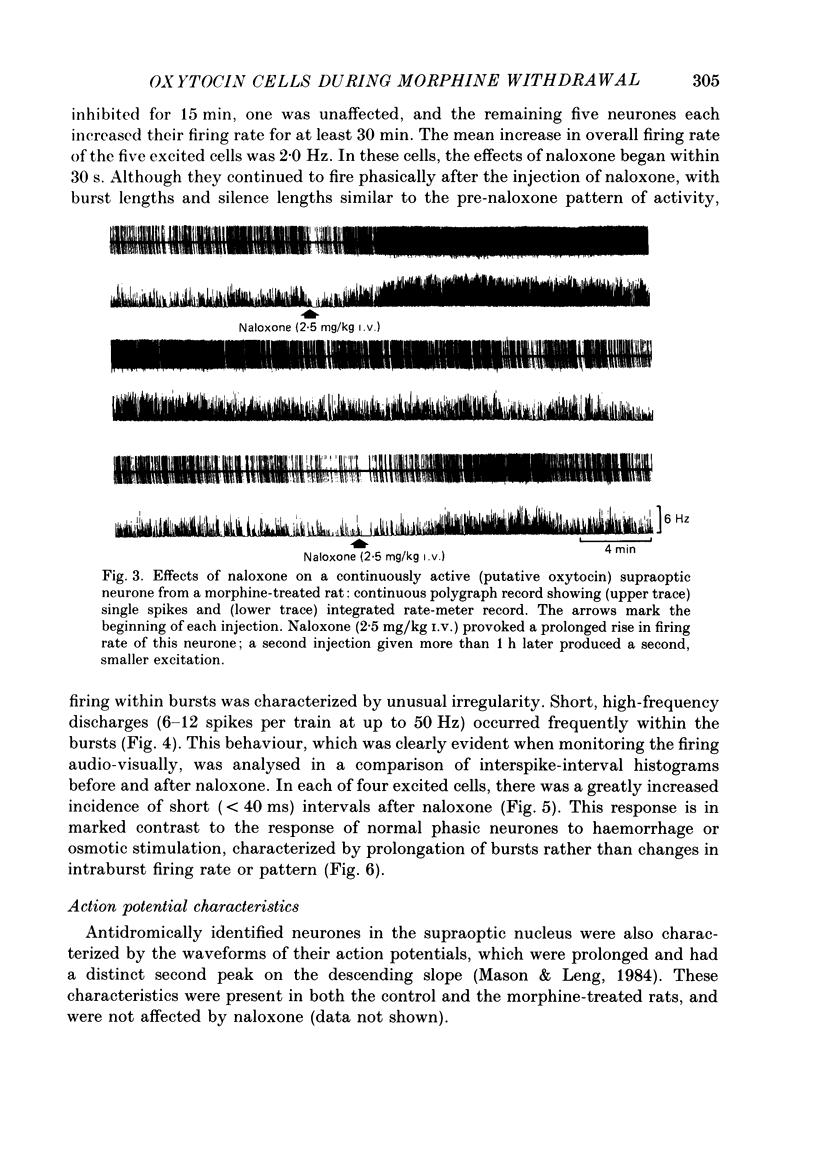







Selected References
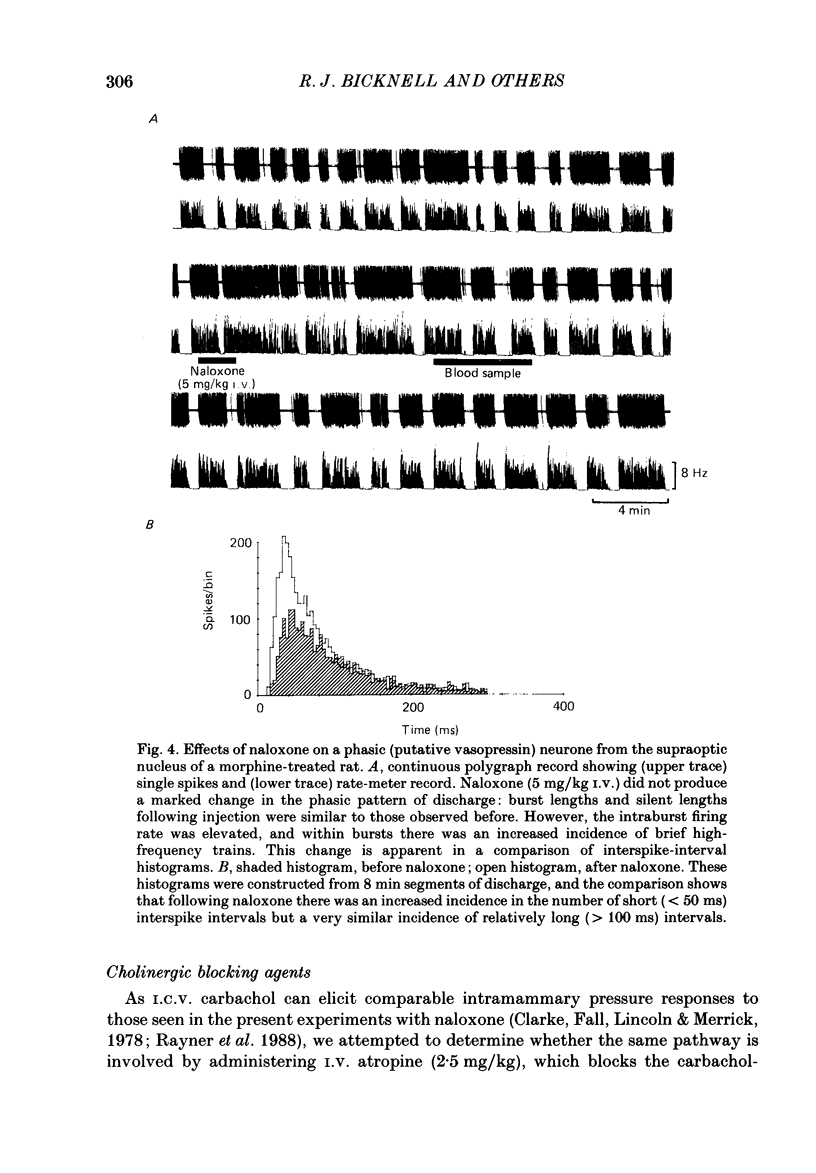
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978 Nov 9;276(5684):186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. J Physiol. 1984 Aug;353:171–185. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Atweh S. F., Kuhar M. J. Distribution and physiological significance of opioid receptors in the brain. Br Med Bull. 1983 Jan;39(1):47–52. doi: 10.1093/oxfordjournals.bmb.a071789. [DOI] [PubMed] [Google Scholar]
- Aziz L. A., Forsling M. L., Woolf C. J. The effect of intracerebroventricular injections of morphine on vasopressin release in the rat. J Physiol. 1981 Feb;311:401–409. doi: 10.1113/jphysiol.1981.sp013592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Brown D., Chapman C., Hancock P. D., Leng G. Reversible fatigue of stimulus-secretion coupling in the rat neurohypophysis. J Physiol. 1984 Mar;348:601–613. doi: 10.1113/jphysiol.1984.sp015128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Chapman C., Leng G. Effects of opioid agonists and antagonists on oxytocin and vasopressin release in vitro. Neuroendocrinology. 1985 Aug;41(2):142–148. doi: 10.1159/000124168. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J. Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J Endocrinol. 1985 Dec;107(3):437–446. doi: 10.1677/joe.0.1070437. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G. Endogenous opiates regulate oxytocin but not vasopressin secretion from the neurohypophysis. Nature. 1982 Jul 8;298(5870):161–162. doi: 10.1038/298161a0. [DOI] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977 Sep;271(1):253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E., Forsling M. L. Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei. J Physiol. 1978 May;278:69–78. doi: 10.1113/jphysiol.1978.sp012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero T. J., Owens D. P., Schmoeker P. F., Meyer E. R. Morphine-induced supersensitivity to the effects of naloxone on luteinizing hormone secretion in the male rat. J Pharmacol Exp Ther. 1983 Apr;225(1):35–41. [PubMed] [Google Scholar]
- Clarke G., Fall C. H., Lincoln D. W., Merrick L. P. Effects of cholinoceptor antagonists on the suckling-induced and experimentally evoked release of oxytocin. Br J Pharmacol. 1978 Jul;63(3):519–527. doi: 10.1111/j.1476-5381.1978.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Patrick G. Differential inhibitory action by morphine on the release of oxytocin and vasopressin from the isolated neural lobe. Neurosci Lett. 1983 Aug 29;39(2):175–180. doi: 10.1016/0304-3940(83)90073-3. [DOI] [PubMed] [Google Scholar]
- Clarke G., Wood P., Merrick L., Lincoln D. W. Opiate inhibition of peptide release from the neurohumoral terminals of hypothalamic neurones. Nature. 1979 Dec 13;282(5740):746–748. doi: 10.1038/282746a0. [DOI] [PubMed] [Google Scholar]
- Collier H. O. Cellular site of opiate dependence. Nature. 1980 Feb 14;283(5748):625–629. doi: 10.1038/283625a0. [DOI] [PubMed] [Google Scholar]
- Cuthbert N. J., Francis D. L., Collier H. O. Adaptation of a neuron to normorphine and clonidine. Biochem Soc Trans. 1983 Jan;11(1):65–68. doi: 10.1042/bst0110065. [DOI] [PubMed] [Google Scholar]
- Dafny N. The hypothalamus exhibits electrophysiologic evidence for morphine tolerance and dependence. Exp Neurol. 1982 Jul;77(1):66–77. doi: 10.1016/0014-4886(82)90143-1. [DOI] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley J. C., Maderdrut J. L., Petrusz P. The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol. 1981 Jun 1;198(4):541–565. doi: 10.1002/cne.901980402. [DOI] [PubMed] [Google Scholar]
- French E. D., Zieglgänsberger W. The excitatory response of in vitro hippocampal pyramidal cells to normorphine and methionine-enkephalin may be mediated by different receptor populations. Exp Brain Res. 1982;48(2):238–244. doi: 10.1007/BF00237219. [DOI] [PubMed] [Google Scholar]
- Fry J. P., Herz A., Zieglgänsberger W. A demonstration of naloxone-precipitated opiate withdrawal on single neurones in the morphine-tolerant/dependent rat brain. Br J Pharmacol. 1980 Mar;68(3):585–592. doi: 10.1111/j.1476-5381.1980.tb14574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar J., Hoffman D. L., Zimmerman E. A. Morphine, beta-endorphin and [D-Ala2] Met-enkephalin inhibit oxytocin release by acetylcholine and suckling. Peptides. 1982 Jul-Aug;3(4):663–668. doi: 10.1016/0196-9781(82)90168-1. [DOI] [PubMed] [Google Scholar]
- Harris M. C. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocrinol. 1979 Jul;82(1):115–125. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Ho Y. W., Mason W. T. Synaptic activation of phasic bursting in rat supraoptic nucleus neurones recorded in hypothalamic slices. J Physiol. 1983 Dec;345:297–317. doi: 10.1113/jphysiol.1983.sp014979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huidobro F., Huidobro-Toro J. Antidiuretic effect of morphine and vasopressin in morphine tolerant and non-tolerant rats, differential effects on urine composition. Eur J Pharmacol. 1979 Oct 26;59(1-2):55–64. doi: 10.1016/0014-2999(79)90024-4. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Iversen S. D., Bloom F. E. Opiate receptors influence vasopressin release from nerve terminals in rat neurohypophysis. Nature. 1980 Mar 27;284(5754):350–351. doi: 10.1038/284350a0. [DOI] [PubMed] [Google Scholar]
- Knepel W., Meyer D. K. The effect of naloxone on vasopressin release from rat neurohypophysis incubated in vitro. J Physiol. 1983 Aug;341:507–515. doi: 10.1113/jphysiol.1983.sp014820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepel W., Nutto D., Anhut H., Hertting G. Naloxone promotes stimulus-evoked vasopressin release in vivo. Eur J Pharmacol. 1980 Aug 8;65(4):449–450. doi: 10.1016/0014-2999(80)90353-2. [DOI] [PubMed] [Google Scholar]
- LaRochelle F. T., Jr, North W. G., Stern P. A new extraction of arginine vasopressin from blood: the use of octadecasilyl-silica. Pflugers Arch. 1980 Aug;387(1):79–81. doi: 10.1007/BF00580849. [DOI] [PubMed] [Google Scholar]
- Leng G., Mansfield S., Bicknell R. J., Dean A. D., Ingram C. D., Marsh M. I., Yates J. O., Dyer R. G. Central opioids: a possible role in parturition? J Endocrinol. 1985 Aug;106(2):219–224. doi: 10.1677/joe.0.1060219. [DOI] [PubMed] [Google Scholar]
- Leng G. The effects of neural stalk stimulation upon firing patterns in rat supraoptic neurones. Exp Brain Res. 1981;41(2):135–145. doi: 10.1007/BF00236603. [DOI] [PubMed] [Google Scholar]
- Martin R., Geis R., Holl R., Schäfer M., Voigt K. H. Co-existence of unrelated peptides in oxytocin and vasopressin terminals of rat neurohypophyses: immunoreactive methionine-enkephalin-, leucine-enkephalin- and cholecystokinin-like substances. Neuroscience. 1983;8(2):213–227. doi: 10.1016/0306-4522(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Leng G. Complex action potential waveform recorded from supraoptic and paraventricular neurones of the rat: evidence for sodium and calcium spike components at different membrane sites. Exp Brain Res. 1984;56(1):135–143. doi: 10.1007/BF00237449. [DOI] [PubMed] [Google Scholar]
- Moore G., Lutterodt A., Burford G., Lederis K. A highly specific antiserum for arginine vasopressin. Endocrinology. 1977 Nov;101(5):1421–1435. doi: 10.1210/endo-101-5-1421. [DOI] [PubMed] [Google Scholar]
- Muehlethaler M., Gaehwiler B. H., Dreifuss J. J. Enkephalin-induced inhibition of hypothalmaic paraventricular neurons. Brain Res. 1980 Sep 15;197(1):264–268. doi: 10.1016/0006-8993(80)90457-6. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Hatton J. D., Bloom F. E. Morphine and opioid peptides reduce paraventricular neuronal activity: studies on the rat hypothalamic slice preparation. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5527–5531. doi: 10.1073/pnas.77.9.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Rayner V. C., Robinson I. C., Russell J. A. Chronic intracerebroventricular morphine and lactation in rats: dependence and tolerance in relation to oxytocin neurones. J Physiol. 1988 Feb;396:319–347. doi: 10.1113/jphysiol.1988.sp016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond D. E., Jr, Krystal J. H. Multiple mechanisms of withdrawal from opioid drugs. Annu Rev Neurosci. 1984;7:443–478. doi: 10.1146/annurev.ne.07.030184.002303. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Joseph S. A. The distribution and cells of origin of ACTH(1-39)-stained varicosities in the paraventricular and supraoptic nuclei. Brain Res. 1982 Jan 28;232(2):365–374. doi: 10.1016/0006-8993(82)90280-3. [DOI] [PubMed] [Google Scholar]
- Sheldrick E. L., Flint A. P. Circulating concentrations of oxytocin during the estrous cycle and early pregnancy in sheep. Prostaglandins. 1981 Oct;22(4):631–636. doi: 10.1016/0090-6980(81)90072-1. [DOI] [PubMed] [Google Scholar]
- Summy-Long J. Y., Keil L. C., Sells G., Kirby A., Chee O., Severs W. B. Cerebroventricular sites for enkephalin inhibition of the central actions of angiotensin. Am J Physiol. 1983 Apr;244(4):R522–R529. doi: 10.1152/ajpregu.1983.244.4.R522. [DOI] [PubMed] [Google Scholar]
- Summy-Long J. Y., Miller D. S., Rosella-Dampman L. M., Hartman R. D., Emmert S. E. A functional role for opioid peptides in the differential secretion of vasopressin and oxytocin. Brain Res. 1984 Sep 10;309(2):362–366. [PubMed] [Google Scholar]
- Summy-Long J. Y., Rosella L. M., Keil L. C. Effects of centrally administered endogenous opioid peptides on drinking behavior, increased plasma vasopressin concentration and pressor response to hypertonic sodium chloride. Brain Res. 1981 Sep 28;221(2):343–357. doi: 10.1016/0006-8993(81)90783-6. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Morita K., North A. Opiates and clonidine prolong calcium-dependent after-hyperpolarizations. Nature. 1981 Nov 12;294(5837):162–163. doi: 10.1038/294162a0. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Noble R., Clarke G. Effects of morphine and D-Ala, D-Leu enkephalin on the electrical activity of supraoptic neurosecretory cells in vitro. Neuroscience. 1983 Sep;10(1):73–81. doi: 10.1016/0306-4522(83)90081-7. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Miller R. J. Opiates, second messengers and cell response. Br Med Bull. 1983 Jan;39(1):53–58. doi: 10.1093/oxfordjournals.bmb.a071791. [DOI] [PubMed] [Google Scholar]
- Whitnall M. H., Gainer H., Cox B. M., Molineaux C. J. Dynorphin-A-(1-8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983 Dec 9;222(4628):1137–1139. doi: 10.1126/science.6648526. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F. W., Pool C. W., Sluiter A. A. Enkephalin immunoreactivity in synaptoid elements on glial cells in the rat neural lobe. Neuroscience. 1983;8(2):229–241. doi: 10.1016/0306-4522(83)90062-3. [DOI] [PubMed] [Google Scholar]


