Abstract
Introduction
For patients with rheumatoid arthritis (RA) unresponsive to first-line biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs), the selection of second-line b/tsDMARDs is crucial to prevent progression to difficult-to-treat rheumatoid arthritis (D2TRA). However, indicators for selection are lacking. This study aimed to identify optimal second-line b/tsDMARDs among the phase III treatment strategies based on European League Against Rheumatism (EULAR) RA management recommendations.
Methods
A total of 687 RA patients treated with second-line b/tsDMARDs (tumor necrosis factor inhibitor (n = 246), interleukin-6 receptor inhibitor [n = 195], cytotoxic T-lymphocyte-associated protein 4 immunoglobulin [n = 119], and Janus kinase inhibitor [n = 127]) were enrolled between October 2013 and April 2023. Rates of patients achieving Clinical Disease Activity Index (CDAI) remission and CDAI low disease activity (LDA), changes in CDAI, persistence rates, and adverse events within 24 weeks after treatment initiation were compared among the four groups. Propensity score-based inverse probability of treatment weighting (PS-IPTW) was used to minimize selection bias.
Results
After PS-IPTW adjustment, the Janus kinase inhibitor (JAKi) group had the highest persistence rate among the four groups. At 24 weeks, the JAKi group showed the greatest improvement in CDAI and the highest CDAI remission rate. Among patients treated with JAKi as second-line b/tsDMARDs, upadacitinib (UPA) was most likely to achieve CDAI remission at 24 weeks. The comparison between the UPA group (n = 32) and the non-UPA JAKi group (tofacitinib and baricitinib [n = 95]) showed comparable persistence rates but significantly lower CDAI scores and higher CDAI remission rate at 24 weeks in the UPA group. No significant difference was noted in the overall incidence of adverse events among the four groups treated with b/tsDMARDs or between the groups treated with JAKi.
Conclusions
Selecting JAKi, especially UPA, may effectively improve the disease activity for RA patients unresponsive to first-line b/tsDMARDs. Further large-scale studies are needed to clarify the efficacy and safety of UPA.
Trial Registration
FIRST registry (approval number#04-23): October 2013, retrospectively registered.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-025-00747-9.
Keywords: Rheumatoid arthritis, Difficult-to-treat rheumatoid arthritis, EULAR RA management recommendations, Janus kinase inhibitor, Upadacitinib
Key Summary Points
| Why carry out this study? |
| In the real-world management of biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) for rheumatoid arthritis (RA), bDMARDs are frequently preferred as first-line therapy due to safety concerns of Janus kinase inhibitor (JAKi). |
| Although JAKi are more commonly considered for the second-line therapy, the efficacy and safety of both bDMARDs and JAKi in patients with RA unresponsive to first-line b/tsDMARDs remain unclear. |
| Second-line therapy is an essential phase in preventing progression to difficult-to-treat rheumatoid arthritis (D2TRA), and we compared the efficacy and safety of second-line b/tsDMARDs from our registry. |
| What was learned from the study? |
| In RA patients unresponsive to first-line b/tsDMARDs, JAKi as second-line therapy resulted in the most rapid reduction in Clinical Disease Activity Index (CDAI) at 4 weeks after initiation of b/tsDMARDs and achieved the highest CDAI remission rate at 24 weeks. Among the three types of JAKi (tofacitinib, baricitinib, upadacitinib), upadacitinib (UPA) was most effective in achieving CDAI remission in RA patients unresponsive to first-line b/tsDMARDs. |
| Selecting JAKi, especially UPA, may effectively improve the disease activity for RA patients unresponsive to first-line b/tsDMARDs. Further large-scale studies are needed to clarify the efficacy and safety of UPA. |
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by the excessive production of proinflammatory cytokines, leading to arthritis, bone destruction, and systemic organ damage [1, 2]. In 1988, methotrexate (MTX) was approved for the treatment of RA, and many biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have become available [3]. Despite advancements in treatment options, disease activity remains dysregulated in some RA patients. Recently, patients with RA unresponsive to two or more b/tsDMARDs with different mechanisms have been diagnosed with difficult-to-treat rheumatoid arthritis (D2TRA), which presents a significant treatment problem for RA [4, 5]. Patients with long disease duration and accumulated joint damage are more likely to develop D2TRA. These findings indicate the importance of early selection of the optimal drug to control disease activity and prevent progression to D2TRA.
In the European League Against Rheumatism Rheumatoid Arthritis management recommendations (EULAR RA management recommendations) [6], the introduction of b/tsDMARDs should be considered in phase II of the treatment algorithm. There are no established precision medicine approaches to determine the most effective b/tsDMARDs for individual patients. Consequently, the medications are selected based on the characteristics of each b/tsDMARD and physicians’ experiences. In addition, previous reports showed that the incidence of malignant tumors and major adverse cardiovascular events (MACE) with Janus kinase inhibitor (JAKi) is non-inferior to that with tumor necrosis factor inhibitors (TNFi), and the safety of JAKi remains a concern compared to other bDMARDs [7]. Since the selection of JAKi should be considered after thorough risk assessment for EULAR RA management recommendations, bDMARDs are often selected more frequently in first-line therapy considering the balance between efficacy and safety. After first-line b/tsDMARDs are ineffective, the phase III treatment strategy is critical in RA treatment because of involvement with progress to D2TRA [5]. However, there is no indicator to guide the selection of second-line b/tsDMARDs, presenting a significant clinical question.
To address this gap, the efficacy and safety of b/tsDMARDs used in second-line therapy were compared from the FIRST registry, a multicenter registry of RA patients treated with b/tsDMARDs. Given the selection biases associated with each b/tsDMARD, propensity score-based inverse probability of treatment weighting (PS-IPTW) was used to minimize these biases for comparison.
Methods
Study Design and Patient Groups
We selected patients who started second-line b/tsDMARDs between October 2013 and April 2023 and were treated for 24 weeks or longer from the FIRST registry. In the inclusion criteria, patients treated with second-line b/tsDMARDs were enrolled after primary or secondary failure of first-line b/tsDMARDs. Patients who switched medications due to adverse events or by personal request were excluded. Additionally, patients who received high-dose glucocorticoids (GCs) for acute exacerbation of interstitial lung disease or extra-articular symptoms such as interstitial lung disease (ILD) before starting b/tsDMARDs were excluded. The diagnosis of RA was based on the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria [8] or the 1987 ACR classification criteria [9]. This study was conducted in accordance with the STROBE guidelines for observational studies (Supplementary Table S1).
Treatment with b/tsDMARDs
According to EULAR RA management recommendations [5], b/tsDMARDs were prescribed to patients who had difficulty controlling disease activity even with standard doses of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), including MTX and to those unable to tolerate csDMARDs. Before initiating b/tsDMARDs treatment, all patients were admitted to the Hospital of the University of Occupational and Environmental Health, Japan, and underwent urine, blood, coagulation function, fecal occult blood tests, and whole-body computed tomography (CT) for screening of complications such as infections and malignant tumors [10]. Patients were also assessed for cardiovascular risk factors (smoking, obesity, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, age, sex, and family history). Patients with diabetes mellitus, hypertension, obesity, or dyslipidemia were examined and treated by endocrinologists. Surgical specialists re-examined patients with a history of malignant tumors to confirm the absence of relapse and other conditions before starting b/tsDMARDs, ensuring the safety of treatment. Subsequently, patients were informed about the risks associated with b/tsDMARDs and alternative treatment options. After obtaining their informed consent, we introduced b/tsDMARDs. For the selection of b/tsDMARDs, several rheumatologists comprehensively contemplated treatment options and selected b/tsDMARDs from those available in each period. Regarding doses of b/tsDMARDs, administration of the drug was started at maximally tolerable doses considering hepatic or renal function. The initial dose was reduced as necessary in patients with hepatic or renal dysfunction. Supplementary Table S2 shows the type and dosage of each drug.
Outcomes
The primary outcome was the rate of achieving Clinical Disease Activity Index (CDAI) remission at 24 weeks after treatment initiation in patients treated with second-line b/tsDMARDs according to the classes of b/tsDMARDs (TNFi, Interleukin-6 receptor inhibitor [IL-6Ri], cytotoxic T lymphocyte-associated protein 4 immunoglobulin [CTLA4Ig], and JAKi) [11]. The secondary outcomes were comparisons of the rate of achieving CDAI-low disease activity (LDA) and remission at 24 weeks after treatment initiation, CDAI scores, laboratory data, quality of life (QoL), persistence rate of b/tsDMARDs at 24 weeks, and incidence of adverse events according to the drug classes. Multivariate analysis was conducted to identify the drug class that achieved the highest CDAI remission rate, and comparisons were performed as described above. The evaluations by Simplified Disease Activity Index (SDAI) and Disease Activity Score in 28 joints (DAS28) were not performed since this study includes IL-6Ri and JAKi, which significantly reduce inflammatory markers such as C-reactive protein (CRP) and erythrocyte sedimentation rates (ESRs).
CDAI-LDA was defined as a CDAI score of ≤ 10.0, and CDAI remission was defined as a CDAI score of ≤ 2.8. Urine and blood tests were performed on admission and at regular outpatient visits. Simultaneously, the Health Assessment Questionnaire-Disability Index (HAQ-DI) and the European Questionnaire 5 Dimension (EQ5D) were also administered to the patients to evaluate their QoL. All adverse events that occurred within 24 weeks after treatment initiation were recorded for safety evaluation, and adverse events and abnormal laboratory test results were evaluated with the Common Terminology for Adverse Events (CTCAE) grade version 5.0 by the National Institute of Health (NIH).
Propensity Score-Based Inverse Probability of Treatment Weighting (PS-IPTW)
To calculate the propensity score (PS), the objective variables were the classes of b/tsDMARDs, and explanatory variables were sex, age, disease duration, duration between first-line b/tsDMARD failure and initiation of second-line therapy, use of MTX, use of GCs, swollen joints (SJ), tender joints (TJ), patient’s global assessment (PGA), evaluator’s global assessment (EGA), HAQ-DI, serum CRP level, and rheumatoid factor (RF) positivity rate (area under the curve [AUC] = 0.773). Each patient was weighted by the formula "ratio of patients treated with each b/tsDMARD to all patients/PS."
Statistical Analysis
Patient characteristics are expressed as mean ± standard deviation (SD) or number (%) of patients. Comparisons of values before and after treatment in each group were performed with a paired t test. Pearson’s χ2 test was used for the comparison of categorical variables. Bonferroni correction was used to adjust for multiple comparisons of categorical variables. Student’s t test or Mann–Whitney’s U test was performed for the comparisons of two groups, and one-way analysis of variance (ANOVA) was used for the comparison of three or more groups. When the p value was less than 0.05, Dunnett’s test was performed with reference to one group as a multiple testing adjustment. Tukey’s honest significant difference (HSD) test, which performs pairwise comparisons in a round-robin manner, was not conducted owing to the outcomes of this study. Kaplan–Meier method was used to assess the persistence rates and the differences between groups were analyzed by the log-rank test. As the missing values at 24 weeks after the introduction of b/tsDMARDs were 1.0% of each group and missing completely at random (MCAR), the last observation carried forward (LOCF) was used to analyze the data. Furthermore, multiple imputation was performed for missing data as part of the sensitivity analysis. The predictor variables included the classes of b/tsDMARDs, age, sex, disease duration, use of MTX, use of GCs, TJ, SJ, PGA, EGA, CRP, HAQ-DI, and RF positivity. To minimize the imputation, only missing values were imputed. SPSS software V.29.0 (IBM Corporation, Armonk, NY, USA) was used for analysis with 100 imputed datasets. Statistical significance was set at p value < 0.05. All analyses were conducted using JMP V.15.0 (SAS Institute, Cary, NC, USA) and SPSS software V.29.0. The statistical power calculation for the post hoc analysis was performed using the G*Power software (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany).
Ethics Approval
This study was approved by the ethics review board of the University of Occupational and Environmental Health, Japan (approval number #04-23). The FIRST registry includes RA patients who initiated treatment with b/tsDMARDs. Informed consent was obtained from all patients who have consented to the FIRST registry.
Results
Comparison of Efficacy of Second-Line b/tsDMARDs After Adjustment by PS-IPTW
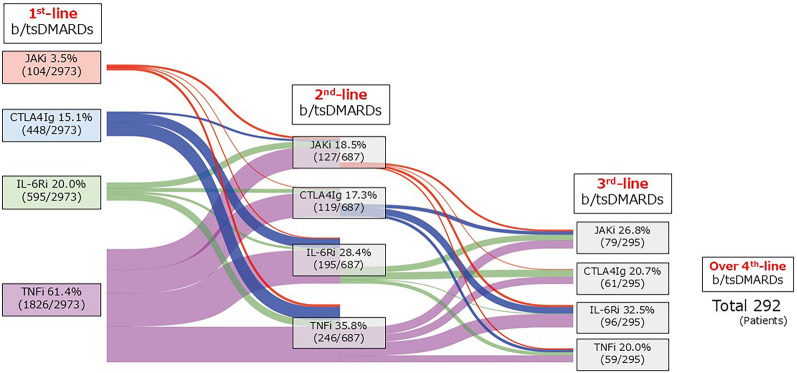
Overall, 2973 patients with RA were initiated on the first-line b/tsDMARDs during the observation period (Fig. 1). The majority of patients received TNFi (61.4%), while only 3.5% of patients were treated with JAKi. Among these, 687 patients were started on second-line b/tsDMARDs because of an inadequate response to first-line therapy, and these cases were included in this study (TNFi, 246 patients; IL-6Ri, 195 patients; CTLA4Ig, 119 patients; JAKi, 127 patients) (Supplementary Table S3). Additionally, 295 patients received third-line b/tsDMARDs owing to the inefficacy of second-line therapy, and 292 patients received b/tsDMARDs as fourth-line therapy or beyond. The TNFi group had a high concomitant rate of MTX. The IL-6Ri group had high serum CRP levels and high ESRs. The CTLA4Ig group tended to include seropositive patients with long disease duration. Patients in the JAKi group had a short disease duration.
Fig. 1.
Sankey diagram of RA patients treated with b/tsDMARDs from October 2013 to April 2023. Trends in the number of RA patients initiating b/tsDMARDs in the FIRST Registry from October 2013 to April 2023. RA rheumatoid arthritis, b/tsDMARDs biologic/targeted synthetic disease-modifying antirheumatic drugs, TNFi tumor necrosis factor inhibitor, IL-6Ri interleukin-6 receptor inhibitor, CTLA4Ig cytotoxic T lymphocyte-associated protein 4-immunoglobulin, JAKi Janus kinase inhibitor
The persistence rate at 24 weeks after introducing each b/tsDMARD was 79.3% for TNFi, 87.2% for IL-6Ri, 84.9% for CTLA4Ig, and 91.3% for JAKi (Supplementary Figure S1A). The most common reason for discontinuation within 24 weeks was ineffectiveness for all b/tsDMARDs (Supplementary Figure S1B). At 24 weeks after the introduction of b/tsDMARDs, CDAI scores, SDAI scores, HAQ-DI, EQ-5D, CRP, ESR, and matrix metalloproteinase 3 (MMP-3) levels all improved from the baseline in each group (Supplementary Figure S1C and Supplementary Table S4).
Due to significant differences in patient characteristics, direct comparisons among these four groups were not feasible. Therefore, PS-IPTW adjustment was employed to minimize selection bias. Table 1 presents the adjusted patient characteristics for all groups. No significant difference was observed in any patient characteristics, with standardized differences all below 0.1, indicating adequate variable balance.
Table 1.
Patient characteristics in the second-line b/tsDMARDs groups after PS-IPTW adjustment
| After PS-IPTW | |||||
|---|---|---|---|---|---|
| Second-line b/tsDMARDs | |||||
| TNFi (N = 251) | IL-6Ri (N = 188) | CTLA4Ig (N = 118) | JAKi (N = 126) | p value | |
| Sex, n (% female) | 212 (84.7%) | 159 (84.4%) | 101 (85.4%) | 107 (84.7%) | 0.996 |
| Age (years) | 61.5 ± 16.3 | 61.5 ± 14.0 | 63.8 ± 11.8 | 60.1 ± 12.4 | 0.440 |
| Disease duration (month) | 116.6 ± 109.9 | 122.4 ± 117.6 | 121.4 ± 122.8 | 115.5 ± 91.8 | 0.923 |
| Duration between first-line b/tsDMARD failure and initiation of second-line therapy (month) | 12.6 ± 21.5 | 14.8 ± 26.9 | 13.9 ± 19.9 | 14.1 ± 22.4 | 0.786 |
| Steinbrocker stage | 2.3 ± 1.0 | 2.3 ± 0.9 | 2.2 ± 1.0 | 2.2 ± 0.9 | 0.870 |
| MTX use at baseline | |||||
| n (%) | 163 (65.0%) | 123 (65.4%) | 80 (67.3%) | 84 (66.5%) | 0.972 |
| Dose (mg/w) | 7.5 ± 6.3 | 7.1 ± 6.1 | 7.5 ± 6.2 | 7.7 ± 6.3 | 0.866 |
| Glucocorticoid use at baseline | |||||
| n (%) | 47 (18.8%) | 36 (19.2%) | 20 (17.5%) | 22 (17.5%) | 0.938 |
| Dose (mg/day) | 1.1 ± 3.2 | 1.0 ± 2.6 | 1.0 ± 2.6 | 1.0 ± 2.9 | 0.982 |
| 28-tender joint count | 8.4 ± 6.5 | 7.8 ± 6.0 | 8.6 ± 6.6 | 8.2 ± 6.5 | 0.681 |
| 28-swollen joint count | 6.2 ± 5.1 | 6.3 ± 4.9 | 6.3 ± 5.1 | 6.3 ± 5.4 | 0.995 |
| PGA, VAS 0–100 mm | 49.1 ± 25.5 | 51.0 ± 23.7 | 52.9 ± 23.2 | 48.6 ± 25.2 | 0.431 |
| EGA, VAS 0–100 mm | 40.8 ± 20.2 | 39.8 ± 18.4 | 39.7 ± 18.0 | 44.1 ± 22.8 | 0.224 |
| Pain VAS, VAS 0–100 mm | 49.6 ± 26.9 | 52.2 ± 24.9 | 51.6 ± 25.4 | 49.6 ± 25.6 | 0.696 |
| CDAI | 23.6 ± 12.9 | 23.2 ± 11.1 | 24.2 ± 11.9 | 23.6 ± 13.6 | 0.919 |
| SDAI | 25.1 ± 14.1 | 25.0 ± 12.3 | 25.8 ± 12.4 | 25.4 ± 15.2 | 0.953 |
| HAQ–DI | 1.2 ± 0.9 | 1.1 ± 0.8 | 1.2 ± 0.8 | 1.1 ± 0.8 | 0.823 |
| EQ-5D | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.316 |
| CRP (mg/dl) | 1.5 ± 2.5 | 1.8 ± 2.6 | 1.6 ± 4.4 | 1.8 ± 3.5 | 0.803 |
| ESR (mm/h) | 43.4 ± 29.0 | 46.5 ± 29.3 | 43.9 ± 32.2 | 47.5 ± 36.2 | 0.554 |
| MMP-3 (ng/ml) | 187.1 ± 261.4 | 227.7 ± 295.1 | 149.2 ± 214.3 | 191.1 ± 276.9 | 0.417 |
| Rheumatoid factor (U/ml) | 201.4 ± 393.3 | 190.1 ± 356.5 | 223.4 ± 892.5 | 234.7 ± 528.0 | 0.887 |
| Rheumatoid factor positive, n (%) | 187 (74.7%) | 141 (74.9%) | 93 (78.6%) | 95 (75.6%) | 0.863 |
| Anti-CCP antibody (U/ml) | 379.7 ± 601.1 | 340.0 ± 592.9 | 563.6 ± 1296.1 | 401.7 ± 1093.4 | 0.437 |
| Anti-CCP antibody, n (%) | 179 (71.5%) | 138 (73.5%) | 80 (67.8%) | 94 (75.0%) | 0.608 |
The data are the mean ± SD or the number (%) of patients. The Steinbrocker stage was applied as the radiographic severity classification [29]. The dose of glucocorticoid was converted into a prednisone-equivalent dose. p values were demonstrated for continuous variables by ANOVA and nominal variables by χ-square test among the four groups.
b/tsDMARDs biologic/targeted synthetic disease-modifying antirheumatic drugs, PS-IPTW propensity score-based inverse probability of treatment weighting, RA rheumatoid arthritis, MTX methotrexate, PGA VAS patient’s global assessment of disease activity visual analog scale, EGA VAS evaluator global assessment of disease activity visual analog scale, CDAI Clinical Disease Activity Index, SDAI Simplified Disease Activity Index, HAQ-DI Health Assessment Questionnaire Disability Index, EQ-5D EuroQoL 5 dimension, CRP C-reactive protein, ESR erythrocyte sedimentation rate, MMP-3 matrix metalloproteinase 3, CCP cyclic citrullinated peptide, TNFi tumor necrosis factor inhibitor, IL-6Ri interleukin-6 receptor inhibitor, CTLA4Ig cytotoxic T-lymphocyte antigen 4 immunoglobulin, JAKi Janus kinase inhibitor
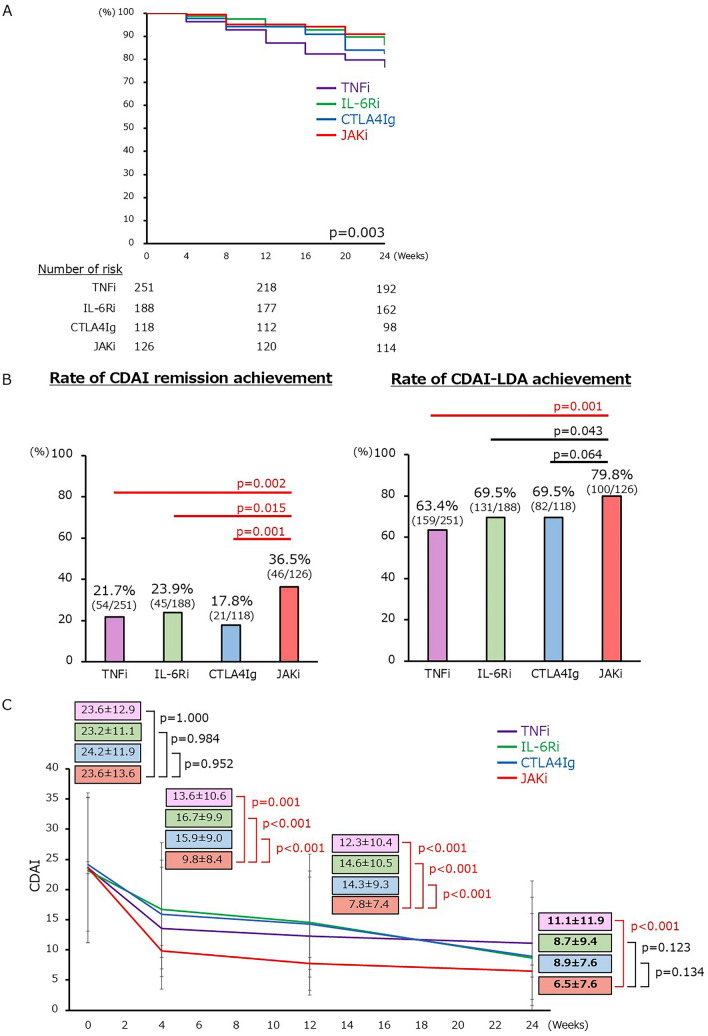
After PS-IPTW adjustment, the 24-week persistence rate differed among the four groups (Fig. 2A and Supplementary Table S5). The JAKi group had the highest persistence rate, significantly higher than the TNFi group. At 24 weeks after treatment initiation, the CDAI remission rate was significantly higher in the JAKi group than in each bDMARD group (Fig. 2B). Even in the sensitivity analysis using the data with missing values imputed through multiple imputation, the CDAI remission rate at week 24 was significantly higher in the JAKi group (JAKi vs. TNFi = 36.8% [47/126] vs. 21.3% [53/251] p = 0.001, JAKi vs. IL-6Ri = 36.8% [47/126] vs. 23.7% [45/188] p = 0.014, JAKi vs. CTLA4Ig = 36.8% [47/126] vs. 17.0% [20/118] p < 0.001). The CDAI-LDA achievement rate was also significantly higher in the JAKi group than in the TNFi group. According to the changes in CDAI scores after the introduction of b/tsDMARDs, the JAKi group had substantially lower CDAI scores at 4 weeks after treatment initiation than each bDMARD group and lower CDAI scores also at 24 weeks (Fig. 2C). The changes in disease activity and HAQ-DI scores from baseline to 24 weeks were most significant in the JAKi group (Supplementary Table S5). The ESR changed more greatly in the JAKi group than in the TNFi and CTLA4Ig groups, whereas the changes in ESR were greater in the IL-6Ri group than in the JAKi group.
Fig. 2.
Comparison of efficacy of second-line b/tsDMARDs after PS-IPTW adjustment. A Persistence rate at 24 weeks after b/tsDMARDs introduction (Kaplan–Meier curves). p values were calculated by log-rank test. B Rate of patients achieving CDAI remission (left) and CDAI-LDA (right) at 24 weeks after b/tsDMARDs. Pearson’s × 2 test was performed for JAKi and each bDMARD. The significance level was adjusted using the Bonferroni correction. C Change in CDAI after introduction of b/tsDMARDs with mean ± SD. Dunnett’s test was performed in comparison with bDMARDs using the reference of JAKi. b/tsDMARDs biologic/targeted synthetic disease-modifying antirheumatic drugs, PS-IPTW propensity score-based inverse probability of treatment weighting, TNFi tumor necrosis factor inhibitor, IL-6Ri interleukin-6 receptor inhibitor, CTLA4Ig cytotoxic T lymphocyte-associated protein 4-immunoglobulin, JAKi Janus kinase inhibitor, CDAI Clinical Disease Activity Index, LDA low disease activity
Next, we analyzed whether b/tsDMARDs used in first-line therapy affected the achievement of CDAI remission at 24 weeks after the introduction in each b/tsDMARD used in second-line therapy (Supplementary Table S6). Neither univariate nor multivariate analysis showed significant differences with the impact of the CDAI remission achievement in each first-line b/tsDMARD.
Comparison of the Safety of b/tsDMARDs Used in Second-Line Therapy
Adverse events leading to discontinuing second-line b/tsDMARDs within 24 weeks after its initiation are shown in Supplementary Figure S2. Severe skin rashes, such as injection site reactions, were more frequent in the TNFi group, while gastrointestinal symptoms were more frequent in the CTLA4Ig and JAKi groups. After adjustment by PS-IPTW, the overall incidence of adverse events was comparable among the four groups (Supplementary Table S7). Among adverse events, gastrointestinal symptoms were more frequent in the JAKi group than in the TNFi group. The profile of adverse events associated with b/tsDMARDs is shown in Supplementary Table S8.
Identification of JAKi Used as Second-Line b/tsDMARDs that is Most Associated with CDAI Remission at 24 Weeks
Next, multivariate analysis was performed to identify factors associated with CDAI remission at 24 weeks in patients with RA who started second-line therapy with JAKi (tofacitinib [TOF], 40 patients; baricitinib [BAR], 55 patients; upadacitinib [UPA], 32 patients) (Table 2). After adjusting for baseline factors (age, sex, disease duration, CDAI, HAQ-DI, and use of MTX), the introduction of UPA was significantly more associated with CDAI remission at 24 weeks than TOF (odds ratio: 5.71, 95%CI: 1.62–20.14, p = 0.007).
Table 2.
JAKi as a second-line option that is most associated with CDAI remission at 24 weeks
| JAKi as second-line b/tsDMARDs (n = 127) | ||||
|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | |||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| TOF | Ref | – | ||
| BAR (TOF reference) | 2.11 (0.78–5.71) | 0.142 | ||
| UPA (TOF reference) | 4.16 (1.43–12.14) | 0.007 | 5.71 (1.62–20.14) | 0.007 |
In the multivariate analysis of factors strongly associated with the achievement of CDAI remission at 24 weeks in RA patients treated with JAKi as second-line b/tsDMARDs, age, sex, disease duration, CDAI, HAQ-DI, and use of MTX at baseline were considered covariates.
b/tsDMARDs biologic/targeted synthetic disease-modifying antirheumatic drugs, JAKi Janus kinase inhibitor, TOF tofacitinib, BAR baricitinib, UPA upadacitinib
We also examined whether first-line b/tsDMARDs were associated with CDAI remission at 24 weeks in each JAKi used as second-line b/tsDMARDs (Supplementary Table S9). Neither univariate nor multivariate analysis showed significant differences with the impact of the CDAI remission achievement at 24 weeks in each first-line b/tsDMARD.
Comparison of Efficacy Between UPA and Non-UPA JAKi Used as Second-Line b/tsDMARDs After Adjustment by PS-IPTW
As UPA might be more optimal for second-line b/tsDMARDs than non-UPA JAKi (TOF and BAR), efficacy was evaluated at 24 weeks in patients with RA who started second-line therapy with UPA group (n = 32) and non-UPA JAKi group (n = 95). The UPA group had a longer disease duration and a higher percentage of patients concomitantly using GC than the non-UPA JAKi group. The concomitant rate of MTX was higher in the non-UPA JAKi group (Table 3 left).
Table 3.
Patient characteristics in the UPA and non-UPA JAKi groups as second-line b/tsDMARDs
| Before PS-IPTW | After PS-IPTW | |||||
|---|---|---|---|---|---|---|
| UPA (N = 32) | Non-UPA JAKi (N = 95) | p value | UPA (N = 30) | Non-UPA JAKi (N = 96) | p value | |
| Sex (female), n (%) | 26 (81.3%) | 79 (83.2%) | 0.805 | 22 (73.9%) | 78 (81.1%) | 0.392 |
| Age (years) | 59.9 ± 11.2 | 55.4 ± 13.2 | 0.086 | 56.5 ± 11.8 | 56.6 ± 12.9 | 0.982 |
| RA duration(month) | 118.2 ± 103.2 | 81.3 ± 73.8 | 0.030 | 94.0 ± 91.8 | 90.6 ± 77.8 | 0.841 |
| Duration between first-line b/tsDMARD failure and initiation of second-line therapy (month) | 10.8 ± 18.0 | 9.5 ± 17.2 | 0.717 | 9.9 ± 17.3 | 9.6 ± 17.8 | 0.932 |
| Steinbrocker stage | 2.1 ± 1.0 | 2.0 ± 0.8 | 0.431 | 2.0 ± 0.9 | 2.0 ± 0.8 | 0.893 |
| MTX use at baseline | ||||||
| n (%) | 20 (62.5%) | 71 (74.7%) | 0.184 | 21 (70.2%) | 68 (70.7%) | 0.958 |
| Dose (mg/w) | 7.0 ± 6.1 | 9.0 ± 6.1 | 0.118 | 8.3 ± 6.1 | 8.3 ± 6.2 | 0.981 |
| Glucocorticoid use at baseline | ||||||
| n (%) | 10 (31.3%) | 12 (12.6%) | 0.016 | 7 (22.0%) | 18 (19.0%) | 0.721 |
| Dose (mg/day) | 1.5 ± 3.1 | 1.0 ± 3.2 | 0.426 | 1.3 ± 3.4 | 1.5 ± 3.8 | 0.872 |
| JAKi as second-line drug | UPA: 32 | TOF: 40, BAR: 55 | UPA: 30 | TOF: 41, BAR: 55 | ||
| First-line b/tsDMARDs |
TNFi: 18 (56.3%) IL-6Ri: 9 (28.1%) CTLA4Ig: 1 (3.1%) JAKi: 4 (12.5%) |
TNFi: 69 (72.6%) IL-6Ri: 15 (15.8%) CTLA4Ig: 7 (7.4%) JAKi: 4 (4.2%) |
||||
| 28-tender joint count | 8.0 ± 6.6 | 9.4 ± 6.7 | 0.328 | 8.9 ± 6.6 | 9.1 ± 6.6 | 0.873 |
| 28-swollen joint count | 5.7 ± 5.7 | 6.7 ± 5.3 | 0.369 | 5.5 ± 5.6 | 6.6 ± 5.3 | 0.354 |
| PGA, VAS 0–100 mm | 52.0 ± 26.6 | 51.8 ± 23.2 | 0.969 | 55.2 ± 27.5 | 53.8 ± 25.6 | 0.804 |
| EGA, VAS 0–100 mm | 50.9 ± 25.8 | 44.4 ± 20.5 | 0.148 | 52.9 ± 24.5 | 47.9 ± 23.8 | 0.326 |
| Pain VAS, VAS 0–100 mm | 54.2 ± 27.9 | 50.4 ± 24.5 | 0.472 | 56.0 ± 27.5 | 53.4 ± 26.3 | 0.642 |
| CDAI | 24.0 ± 14.6 | 25.5 ± 12.6 | 0.586 | 25.2 ± 14.2 | 24.9 ± 12.8 | 0.911 |
| SDAI | 25.2 ± 15.1 | 27.0 ± 14.6 | 0.567 | 26.6 ± 14.6 | 26.4 ± 14.6 | 0.959 |
| HAQ–DI | 1.0 ± 0.9 | 1.2 ± 0.7 | 0.201 | 1.0 ± 0.8 | 1.1 ± 0.7 | 0.336 |
| EQ-5D | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.585 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.939 |
| CRP (mg/dl) | 1.2 ± 2.2 | 1.6 ± 3.7 | 0.634 | 1.4 ± 2.2 | 1.6 ± 3.9 | 0.764 |
| ESR (mm/h) | 36.8 ± 34.1 | 35.2 ± 30.6 | 0.808 | 35.5 ± 33.2 | 36.4 ± 31.9 | 0.886 |
| MMP-3 (ng/ml) | 133.2 ± 165.0 | 193.8 ± 296.5 | 0.282 | 131.6 ± 169.4 | 192.4 ± 289.0 | 0.283 |
| Rheumatoid factor (U/ml) | 229.9 ± 454.3 | 147.7 ± 429.2 | 0.358 | 200.3 ± 438.3 | 201.8 ± 576.0 | 0.989 |
| Rheumatoid factor positive, n (%) | 27 (84.4%) | 65 (68.4%) | 0.081 | 25 (83.5%) | 70 (73.2%) | 0.252 |
| Anti-CCP antibody (U/ml) | 215.2 ± 389.6 | 275.2 ± 789.1 | 0.681 | 206.2 ± 325.2 | 385.1 ± 1046.3 | 0.462 |
| Anti-CCP antibody, n (%) | 23 (71.9%) | 65 (68.4%) | 0.714 | 22 (73.2%) | 67 (69.6%) | 0.712 |
Data are mean ± SD or number (%) of patients. The Steinbrocker stage was applied as the radiographic severity classification [29]. The dose of glucocorticoid was converted into a prednisone-equivalent dose.
PS-IPTW propensity score-based inverse probability of treatment weighting, RA rheumatoid arthritis, MTX methotrexate, PGA VAS patient’s global assessment of disease activity visual analog scale, EGA VAS evaluator global assessment of disease activity visual analog scale, CDAI Clinical Disease Activity Index, SDAI Simplified Disease Activity Index, HAQ-DI Health Assessment Questionnaire Disability Index, EQ-5D EuroQoL 5 dimension, CRP C-reactive protein, ESR erythrocyte sedimentation rate, MMP-3 matrix metalloproteinase 3, CCP cyclic citrullinated peptide, TOF tofacitinib, BAR baricitinib, UPA upadacitinib, TNFi tumor necrosis factor inhibitor, IL-6Ri interleukin-6 receptor inhibitor, CTLA4Ig cytotoxic T-lymphocyte antigen 4 immunoglobulin, JAKi Janus kinase inhibitor
The 24-week persistence rate was 93.8% in the UPA group and 90.5% in the non-UPA JAKi group (Supplementary Figure S3A). The reasons for discontinuation within 24 weeks included ineffectiveness and adverse events (Supplementary Figure S3B). The CDAI score at 24 weeks after the introduction of JAKi was 5.7 ± 7.3 in the UPA group and 8.1 ± 8.1 in the non-UPA JAKi group, showing improvement at 24 weeks in both groups (Supplementary Figure S3C). Supplementary Figure S3D shows the changes in disease activity in both groups. Although 1–3% of patients were in remission at baseline, tapering the GC dose was difficult with first-line b/tsDMARDs, and these patients switched to second-line b/tsDMARDs. The CDAI remission rate at 24 weeks was 46.9% in the UPA group and 25.3% in the non-UPA JAKi group. At 24 weeks after treatment initiation, the values of HAQ-DI, EQ-5D, CRP, ESR, and MMP-3 improved in both groups (Supplementary Table S10).
Table 3 (right) shows the patient characteristics in both groups after PS-IPTW adjustment. No significant difference was observed in any patient characteristics with standardized differences being less than 0.1 for all patient characteristics, showing an appropriate variable balance.
The persistence rate after PS-IPTW adjustment was 93.5% in the UPA group and 88.8% in the non-UPA JAKi group, showing no significant difference (Fig. 3A and Supplementary Table S11). Although the achievement rate of CDAI-LDA at 24 weeks was comparable between the two groups, the CDAI remission rate was significantly higher in the UPA group (UPA vs. non-UPA JAKi: 49.6% vs. 25.3%, p = 0.012) (Fig. 3B). Owing to the small sample size in the subgroup analysis of JAKi, statistical power was calculated using a post hoc analysis. For the primary endpoint, the CDAI remission rate at 24 weeks after adjustment for PS-IPTW, the remission rate in the UPA group (30 patients) was 49.9%, whereas that in the non-UPA JAKi group (96 patients) was 25.4%. With an α error set at 0.05, the effect size was calculated to be 0.45, and the statistical power was determined to be 0.80. Additionally, owing to differences in disease duration and GC use between the UPA and non-UPA JAKi groups prior to PS-IPTW adjustment, a sensitivity analysis was performed using multivariate analysis to assess the influence of UPA use on CDAI remission at 24 weeks, adjusting for disease duration and GC use. UPA use remained associated with CDAI remission at 24 weeks even after adjusting for these factors (UPA use: odds ratio 3.30, 95% CI 1.32–8.24, p = 0.010). The CDAI score at 24 weeks after the introduction of JAKi was significantly lower in the UPA group than in the non-UPA JAKi group (UPA vs. non-UPA JAKi: 5.7 ± 6.7 vs. 8.0 ± 7.8, p = 0.048) (Fig. 3C). Regarding changes in disease activity and laboratory test results from baseline to 24 weeks, CDAI and SDAI scores greatly improved in the UPA group. Still, no statistically significant difference was observed. These findings suggested that UPA might be more effective as second-line b/tsDMARDs for patients with RA.
Fig. 3.
Comparison of efficacy between UPA and non-UPA JAKi as second-line b/tsDMARDs after PS-IPTW adjustment. A Persistence rate at 24 weeks after JAKi introduction (Kaplan–Meier curves). p values were calculated by log-rank test. B Rate of patients achieving CDAI remission (left) and CDAI-LDA (right) at 24 weeks after JAKi. Pearson’s × 2 test was performed for UPA and non-UPA JAKi groups. C Change in CDAI after introduction of JAKi with mean ± SD. p values were calculated by Student’s t test. b/tsDMARDs biologic/targeted synthetic disease-modifying antirheumatic drugs, PS-IPTW propensity score-based inverse probability of treatment weighting, UPA upadacitinib, JAKi Janus kinase inhibitor, CDAI Clinical Disease Activity Index, LDA low disease activity
Comparison of the Safety of UPA and Non-UPA JAKi Used as Second-Line b/tsDMARDs
Significantly few adverse events led to discontinuation within 24 weeks after the JAKi was introduced as second-line b/tsDMARDs (Supplementary Figure S3B). Thus, both groups’ adverse events within 24 weeks were recorded (Supplementary Figure S4). Adverse events, particularly infections and herpes zoster, were more frequent in the UPA group. After PS-IPTW adjustment, we observed no significant difference in the incidence of adverse events between the two groups (Supplementary Table S12). The profile of the adverse events associated with JAKi is presented in Supplementary Table S13.
Discussion
This study demonstrated that JAKi used as second-line b/tsDMARDs might be more effective among the phase III treatment strategies based on EULAR RA management recommendations. Two weeks after the induction of JAKi, the CDAI score was significantly lower in the JAKi group than in the TNFi group, suggesting that JAKi was fast-acting. Previous studies conducted in our department have demonstrated marked improvement in CDAI scores immediately after the induction of JAKi [12, 13]. Some clinical studies have also reported that JAKi was more effective for improving CDAI scores six months after treatment initiation than TNFi [14–16]. Additionally, some previous safety reports have indicated that the incidence of malignant tumors and MACE with JAKi is non-inferior to that with TNFi [7, 17, 18]. In contrast, no differences were detected in the incidence of malignant tumors or MACE among the b/tsDMARDs since all patients underwent screening for malignant tumors and evaluation of cardiovascular and other risk factors before introducing each b/tsDMARD at our institution [10]. The importance of screening before introducing b/tsDMARDs was demonstrated [19, 20].
Furthermore, this study demonstrated that UPA was the most effective among JAKi when used as a second-line b/tsDMARD. Regarding the changes in CDAI scores after the introduction of JAKi, CDAI scores were comparable between the UPA and non-UPA JAKi groups at 4 and 12 weeks after treatment initiation but significantly lower in the UPA group at 24 weeks. Likewise, the CDAI remission rate at 24 weeks was significantly higher in the UPA group. In short, these results revealed UPA to be the most effective among second-line b/tsDMARDs.
The efficacy of JAKi is considered to be associated with broad-spectrum inhibition [21]. JAKi modulates proinflammatory cytokine signaling by directly targeting the JAK-signal transducer downstream of cytokine receptors on immune cells, thereby regulating arthritis and bone destruction [22, 23]. Unlike bDMARDs which only target specific cytokines such as TNF or IL-6, JAKi regulates multiple cytokines associated with the JAK pathway. Among JAKs, JAK1 regulates representative cytokines involved in the pathogenesis of RA. Since UPA, which is second-generation JAKi, is more selective for JAK1 than first-generation JAKi (TOF and BAR), UPA may be more potent for alleviating arthritis and improving visual analog scale scores (VAS) [24]. Furthermore, the fast-acting of JAKi is associated with intracellular action. Although bDMARDs target extracellular molecules such as TNF receptors, IL-6 receptors, and clusters of differentiation 80/86 (CD80/CD86) on the surface of antigen-presenting cells, JAKi have small molecular weights and act intracellularly. Therefore, JAKi is characteristically faster-acting than bDMARDs [25].
This study has several limitations. The observation period was 24 weeks. Owing to the short observation period, outcomes such as long-term remission maintenance and the presence of progression on imaging could not be assessed. Additionally, safety concerns related to long-term observation, including adverse events and the feasibility of continued treatment, could not be evaluated. Second, the number of patients was smaller in the UPA group than in the non-UPA JAKi group. While TOF and BAR, used in the non-UPA JAKi group, were available from 2013 and 2017 in Japan, UPA was just approved in March 2020. Consequently, SD and variability were larger in the UPA group. The association between UPA and CDAI remission in the subgroup analysis should be interpreted with caution owing to the limited number of patients treated with UPA. Third, the historical background greatly varied during the period when each b/tsDMARD was used. This is an observational study conducted over a 10-year period, beginning from the introduction of JAKi in 2013. Various temporal factors are likely to have influenced the results. For instance, JAKi were first explicitly mentioned in the EULAR Recommendations in 2016 [26], and subsequent updates to the guidelines brought about significant changes to the selection of medications. Notably, after the results of the oral surveillance trial were released, physicians preferred bDMARDs other than JAKi as the first-line b/tsDMARDs [7]. Notably, the selection of first-line b/tsDMARDs was predominantly non-JAKi in this study (Fig. 1). Additionally, the coronavirus disease (COVID-19) pandemic, which began in March 2020, had a significant impact on patient management and outcomes [27]. In addition to the direct effects of COVID-19, factors such as restrictions on hospital visits, limitations on outings, adjustments in the use of antirheumatic drugs, and vaccination may have influenced disease activity, drug continuation rates, and adverse events. Moreover, the number of patients with COVID-19 in Japan was lower than that in other countries [28], hindering the ability to perform an accurate analysis accounting for the impact of COVID-19. Although the patient background factors were adjusted as much as possible using the PS-IPTW in this study, these calendar effects could not be completely eliminated. However, we believe that this study provides an answer to a clinical question in medical practice since no previous studies have been conducted on this topic.
Conclusions
In the phase III treatment strategy based on EULAR RA management recommendations, b/tsDMARDs should be selected through a comprehensive analysis of patient characteristics similar to phase II treatment strategy. Additionally, it might be preferable to consider the introduction of JAKi, especially UPA, for early control of disease activity. However, the number of patients treated with UPA was relatively small and the observation period was short in this study. Therefore, further large-scale and long-term studies are needed to clarify the efficacy and safety of UPA.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplementary figure S1 Comparison of efficacy of second-line b/tsDMARDs before PS-IPTW adjustment. (A) Persistence rate at 24 weeks after b/tsDMARDs introduction (Kaplan-Meier curves). (B) Reasons for discontinuation within 24 weeks of b/tsDMARDs introduction (person-years). (C) Change in CDAI after introduction of b/tsDMARDs with mean±SD. b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, PS-IPTW: propensity score-based inverse probability of treatment weighting, TNFi; tumor necrosis factor inhibitor, IL-6Ri; interleukin-6 receptor inhibitor, CTLA4Ig; cytotoxic T lymphocyte associated protein 4 - immunoglobulin, JAKi; janus kinase inhibitor, CDAI; clinical disease activity index; Supplementary figure S2 Reasons for discontinuation within 24 weeks of second-line b/tsDMARDs introduction (person-years). b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, TNFi; tumor necrosis factor inhibitor, IL-6Ri; interleukin-6 receptor inhibitor, CTLA4Ig; cytotoxic T lymphocyte associated protein 4 - immunoglobulin, JAKi; janus kinase inhibitor. Supplementary figure S3 Comparison of efficacy between UPA and Non-UPA JAKi as second-line b/tsDMARDs before PS-IPTW adjustment. (A) Persistence rate at 24 weeks after JAKi introduction (Kaplan-Meier curves). (B) Reasons for discontinuation within 24 weeks of JAKi introduction (person-years). (C) Change in CDAI after introduction of JAKi with mean±SD. (D) Sankey diagram of CDAI after introduction of JAKi. b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, PS-IPTW: propensity score-based inverse probability of treatment weighting, UPA; upadacitinib, JAKi; janus kinase inhibitor, CDAI; clinical disease activity index, HDA; high disease activity, MDA; moderate disease activity, LDA; low disease activity, REM; remissionSupplementary figure S4 Adverse events in UPA and Non-UPA JAKi groups within 24 weeks of induction (person-years). b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, UPA; upadacitinib, JAKi; janus kinase inhibitor(PDF 1371 KB)
Acknowledgements
The authors thank all medical staff at all participating institutions for providing the data, especially Ms. Hiroko Yoshida, Ms. Youko Saitou, and Ms. Machiko Mitsuiki for the data management in the FIRST registry. We also thank Dr. Kazuyoshi Saito at Tobata General Hospital, Dr. Kentaro Hanami at Kitakyusyu General Hospital, Dr. Keisuke Nakatsuka at Fukuoka Yutaka Hospital, and all staff members at Shimonoseki Saiseikai Hospital for their engagement in data collection of the FIRST registry.
Medical Writing/Editorial Assistance
We would like to thank Honyaku Center Inc. for editing and reviewing this manuscript for the English language. No funding or sponsorship was received for the medical writing of this article.
Author Contributions
Substantial contributions to study concept and design: Ryuichiro Kanda, Yusuke Miyazaki, Shingo Nakayamada, and Yoshiya Tanaka. Substantial contributions to acquisition of data: Ryuichiro Kanda, Yusuke Miyazaki, Shunsuke Fukuyo, Satoshi Kubo, Ippei Miyagawa, Ayako Yamaguchi, Yurie Satoh-Kanda, Naoaki Ohkubo, Yasuyuki Todoroki, Hiroaki Tanaka, Masanobu Ueno, Atsushi Nagayasu, Yuya Fujita, Takafumi Aritomi, Katsuhide Kusaka, Hidenori Sakai, Satsuki Matsunaga, Hirotsugu Nohara. Substantial contributions to analysis and interpretation of data: Ryuichiro Kanda, Yusuke Miyazaki. All authors were involved in the drafting and revision of the manuscript, and all authors approved the final version to be published.
Funding
This work was supported in part by a Grant-In-Aid for Scientific Research from the University of Occupational and Environmental Health, Japan, through the University of Occupational and Environmental Health, Japan, (UOEH) for Advanced Research (#19K17919). No funding or sponsorship was received for the publication of this article.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Yusuke Miyazaki has received consulting fees, speaking fees, lecture fees, and/or honorariums from AstraZeneca, GlaxoSmithKline, Bristol-Myers, Astellas, Asahi-kasei, AbbVie, Chugai, Sanofi, Eisai, Eli Lilly, and Boehringer Ingelheim. Shingo Nakayamada has received consulting fees, speaking fees, lecture fees, and/or honorariums from AstraZeneca, GlaxoSmithKline, Pfizer, Bristol-Myers, Astellas, Asahi-kasei, AbbVie, Chugai, Sanofi, Eisai, Gilead Sciences, Mitsubishi-Tanabe, Janssen, Eli Lilly, Boehringer Ingelheim and Ayumi. Satoshi Kubo has received speaking fees from Eli Lilly, GlaxoSmithKline, Bristol-Myers, AbbVie, Eisai, Pfizer, AstraZeneca and also research grants from Daiichi-Sankyo, AbbVie, Boehringer Ingelheim, and Astellas. Masanobu Ueno has received speaking fees from GlaxoSmithKline. Yoshiya Tanaka has received speaking fees and/or honorariums from Gilead Sciences, AbbVie, Boehringer Ingelheim, Eli Lilly, Mitsubishi-Tanabe, Chugai, Amgen, YL Biologics, Eisai, Astellas, Bristol-Myers, and AstraZeneca; received research grants from Asahi-Kasei, AbbVie, Chugai, Mitsubishi-Tanabe, Eisai, Takeda, Corrona, Daiichi-Sankyo, Kowa, and Boehringer Ingelheim; and received consultant fees from Eli Lilly, Daiichi-Sankyo, Taisho, Ayumi, Sanofi, GlaxoSmithKline, and AbbVie. Ryuichiro Kanda, Shunsuke Fukuyo, Ippei Miyagawa, Ayako Yamaguchi, Yurie Satoh-Kanda, Naoaki Ohkubo, Yasuyuki Todoroki, Hiroaki Tanaka, Atsushi Nagayasu, Yuya Fujita, Takafumi Aritomi, Katsuhide Kusaka, Hidenori Sakai, Satsuki Matsunaga, and Hirotsugu Nohara have nothing to disclose. Yoshiya Tanaka is an Editorial Board member of Rheumatology and Therapy. Yoshiya Tanaka was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This study was approved by the ethics review board of the University of Occupational and Environmental Health, Japan (approval number #04-23). The FIRST registry includes RA patients who initiated treatment with b/tsDMARDs. Informed consent was obtained from all patients who have consented to the FIRST registry.
References
- 1.Gravallese EM, Firestein GS. Rheumatoid arthritis—common origins, divergent mechanisms. N Engl J Med. 2023;388:529–42. [DOI] [PubMed] [Google Scholar]
- 2.Schett G, Tanaka Y, Isaacs JD. Why remission is not enough: underlying disease mechanisms in RA that prevent cure. Nat Rev Rheumatol. 2021;17(3):135–44. [DOI] [PubMed] [Google Scholar]
- 3.Burmester GR, Bijlsma JWJ, Cutolo M, et al. Managing rheumatic and musculoskeletal diseases—past, present and future. Nat Rev Rheumatol. 2017;13(7):443–8. [DOI] [PubMed] [Google Scholar]
- 4.Nagy G, Roodenrijs NMT, Welsing PM, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(1):31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochi S, Sonomoto K, Nakayamada S, et al. Preferable outcome of Janus kinase inhibitors for a group of difficult-to-treat rheumatoid arthritis patients: from the FIRST Registry. Arthritis Res Ther. 2022;24(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. [DOI] [PubMed] [Google Scholar]
- 7.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–26. [DOI] [PubMed] [Google Scholar]
- 8.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8. [DOI] [PubMed] [Google Scholar]
- 9.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 10.Miyata H, Sonomoto K, Fukuyo S, et al. Computed tomography for malignancy screening in patients with rheumatoid arthritis before initiation of disease-modifying antirheumatic drug. Rheumatology (Oxford). 2023;62(10):3339–49. [DOI] [PubMed] [Google Scholar]
- 11.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki Y, Nakano K, Nakayamada S, et al. Efficacy and safety of tofacitinib versus baricitinib in patients with rheumatoid arthritis in real clinical practice: analyses with propensity score-based inverse probability of treatment weighting. Ann Rheum Dis. 2021;80(9):1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubo S, Miyazaki Y, Amano K, et al. Sustained remission following the discontinuation of tofacitinib in patients with rheumatoid arthritis (XANADU study): an open-label randomised study. RMD Open. 2023;9(2): e003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–800. [DOI] [PubMed] [Google Scholar]
- 15.Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–62. [DOI] [PubMed] [Google Scholar]
- 16.Kubo S, Nakayamada S, Tanaka Y. Baricitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2016;12(9):911–9. [DOI] [PubMed] [Google Scholar]
- 17.Clarke B, Yates M, Adas M, et al. The safety of JAK-1 inhibitors. Rheumatology (Oxford). 2021;60(Suppl 2):ii24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–43. [DOI] [PubMed] [Google Scholar]
- 19.Szekanecz Z, Buch MH, Charles-Schoeman C, et al. Efficacy and safety of JAK inhibitors in rheumatoid arthritis: update for the practising clinician. Nat Rev Rheumatol. 2024;20(2):101–15. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka Y. Recent progress in treatments of rheumatoid arthritis: an overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology (Oxford). 2021;60(Suppl 6):vi12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–62. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Luo Y, O’Shea JJ, et al. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo S, Nakayamada S, Tanaka Y. JAK inhibitors for rheumatoid arthritis. Expert Opin Investig Drugs. 2023;32(4):333–44. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y. A review of upadacitinib in rheumatoid arthritis. Mod Rheumatol. 2020;30(5):779–87. [DOI] [PubMed] [Google Scholar]
- 25.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. [DOI] [PubMed] [Google Scholar]
- 26.Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawakami Y, Nojiri S, Nakamoto D, et al. Novel indicator for the spread of new coronavirus disease 2019 and its association with human mobility in Japan. Sci Rep. 2023;13(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc. 1949;140:659–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplementary figure S1 Comparison of efficacy of second-line b/tsDMARDs before PS-IPTW adjustment. (A) Persistence rate at 24 weeks after b/tsDMARDs introduction (Kaplan-Meier curves). (B) Reasons for discontinuation within 24 weeks of b/tsDMARDs introduction (person-years). (C) Change in CDAI after introduction of b/tsDMARDs with mean±SD. b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, PS-IPTW: propensity score-based inverse probability of treatment weighting, TNFi; tumor necrosis factor inhibitor, IL-6Ri; interleukin-6 receptor inhibitor, CTLA4Ig; cytotoxic T lymphocyte associated protein 4 - immunoglobulin, JAKi; janus kinase inhibitor, CDAI; clinical disease activity index; Supplementary figure S2 Reasons for discontinuation within 24 weeks of second-line b/tsDMARDs introduction (person-years). b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, TNFi; tumor necrosis factor inhibitor, IL-6Ri; interleukin-6 receptor inhibitor, CTLA4Ig; cytotoxic T lymphocyte associated protein 4 - immunoglobulin, JAKi; janus kinase inhibitor. Supplementary figure S3 Comparison of efficacy between UPA and Non-UPA JAKi as second-line b/tsDMARDs before PS-IPTW adjustment. (A) Persistence rate at 24 weeks after JAKi introduction (Kaplan-Meier curves). (B) Reasons for discontinuation within 24 weeks of JAKi introduction (person-years). (C) Change in CDAI after introduction of JAKi with mean±SD. (D) Sankey diagram of CDAI after introduction of JAKi. b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, PS-IPTW: propensity score-based inverse probability of treatment weighting, UPA; upadacitinib, JAKi; janus kinase inhibitor, CDAI; clinical disease activity index, HDA; high disease activity, MDA; moderate disease activity, LDA; low disease activity, REM; remissionSupplementary figure S4 Adverse events in UPA and Non-UPA JAKi groups within 24 weeks of induction (person-years). b/tsDMARDs; biologic/targeted synthetic disease-modifying antirheumatic drugs, UPA; upadacitinib, JAKi; janus kinase inhibitor(PDF 1371 KB)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.