Abstract
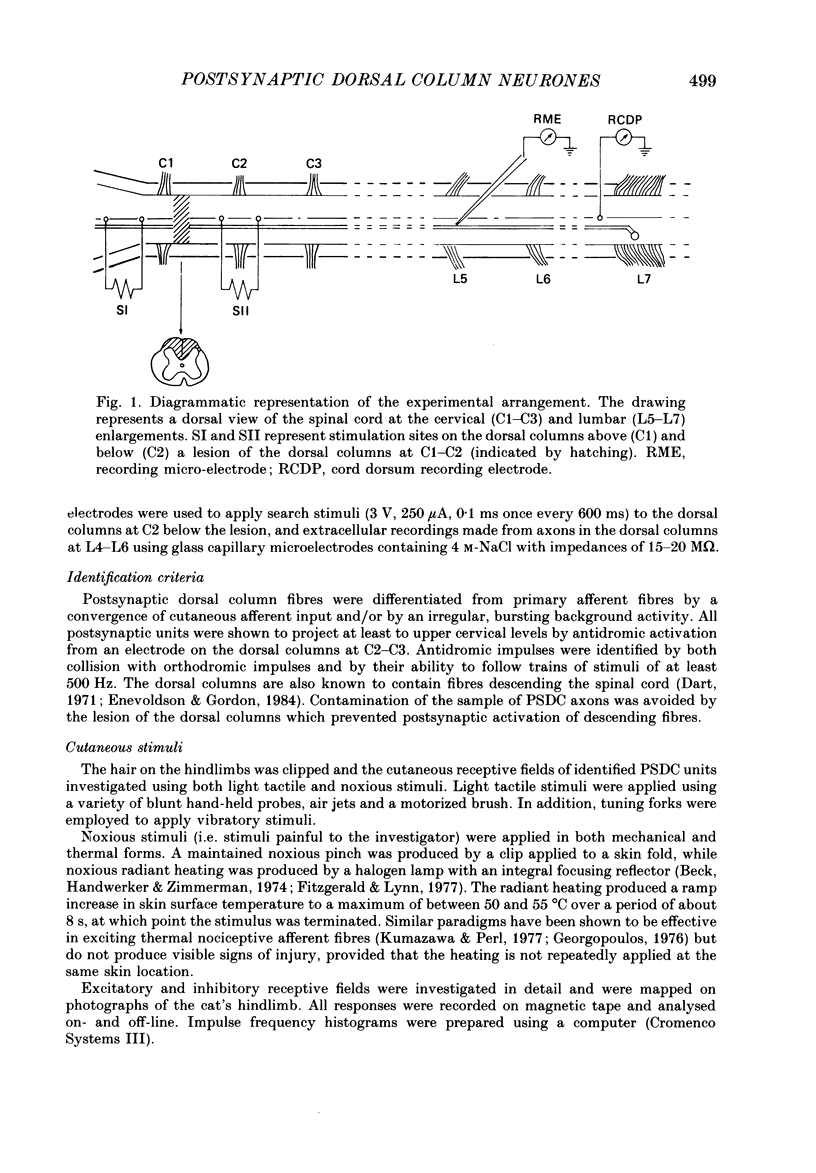
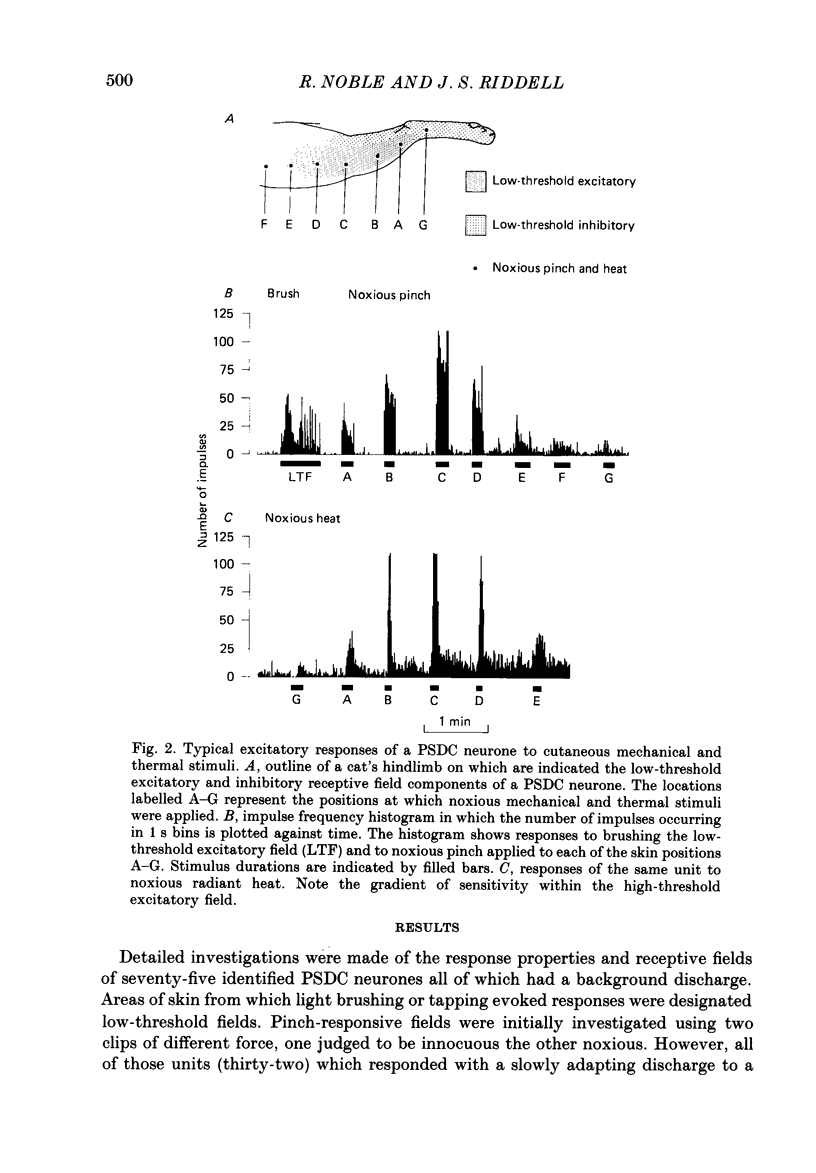
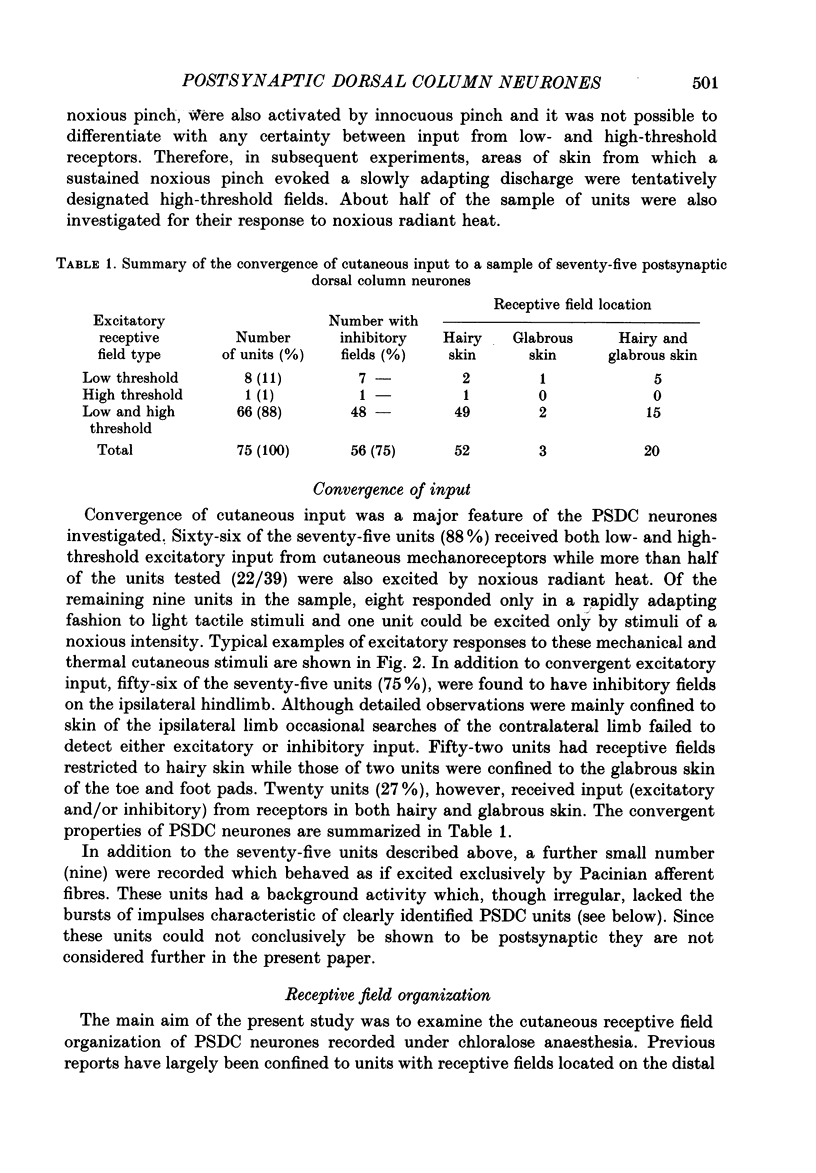
1. In chloralose-anaesthetized cats single-unit microelectrode recordings were made from axons in the dorsal columns, at the lumbar level, identified as belonging to the postsynaptic dorsal column (PSDC) system. 2. Excitatory and inhibitory receptive field arrangements of a sample of seventy-five PSDC neurones were examined in detail using natural cutaneous stimuli. 3. The sample was characterized by a high degree of convergent input: 80% of units were activated by both light tactile and noxious mechanical stimuli and more than half of those examined were excited by noxious radiant heat. In addition, three-quarters of the units had inhibitory receptive fields on the ipsilateral limb. 4. Twenty-three units (27%) were influenced by input from areas of both hairy and glabrous skin covering the foot and distal limb. Neurones in this group had complex receptive fields, many of which occupied several discontinuous areas of skin. Background and evoked activity of these units could frequently be inhibited by light tactile and/or noxious stimuli. Their inhibitory receptive fields occupied small areas of skin overlapping or adjacent to excitatory fields. 5. Fifty-two units (73%) had receptive fields restricted to areas of hairy skin on the thigh and upper hindlimb. Half the units in this group had coextensive low- and high-threshold excitatory areas but about one-third had a concentric receptive field organization; a high-threshold excitatory component extending beyond, or around, a central low-threshold area. The discharge of these units could be inhibited only by light tactile stimuli. Their inhibitory receptive fields covered extensive areas of skin, sometimes completely surrounding the excitatory field. 6. The complex receptive field arrangements observed for neurones of the postsynaptic dorsal column system are discussed in relation to previous observations on dorsal horn neurones of other ascending tracts.
Full text
PDF

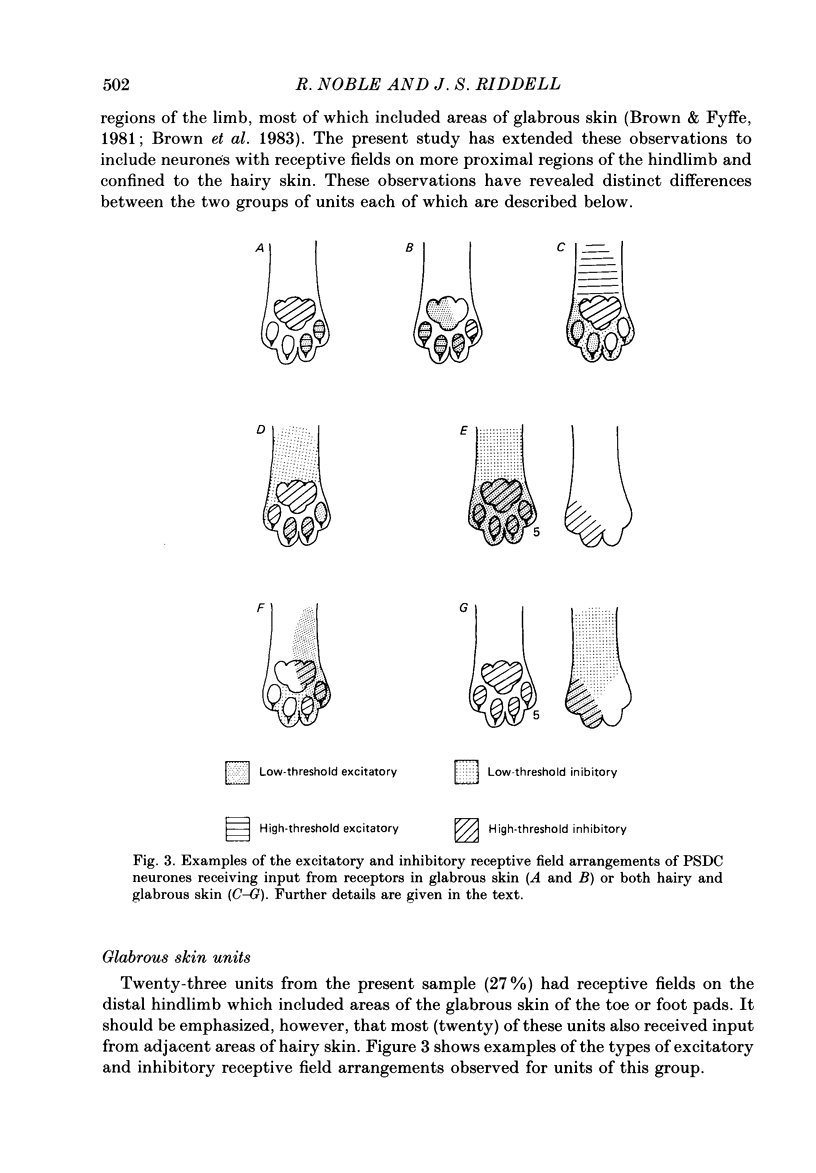
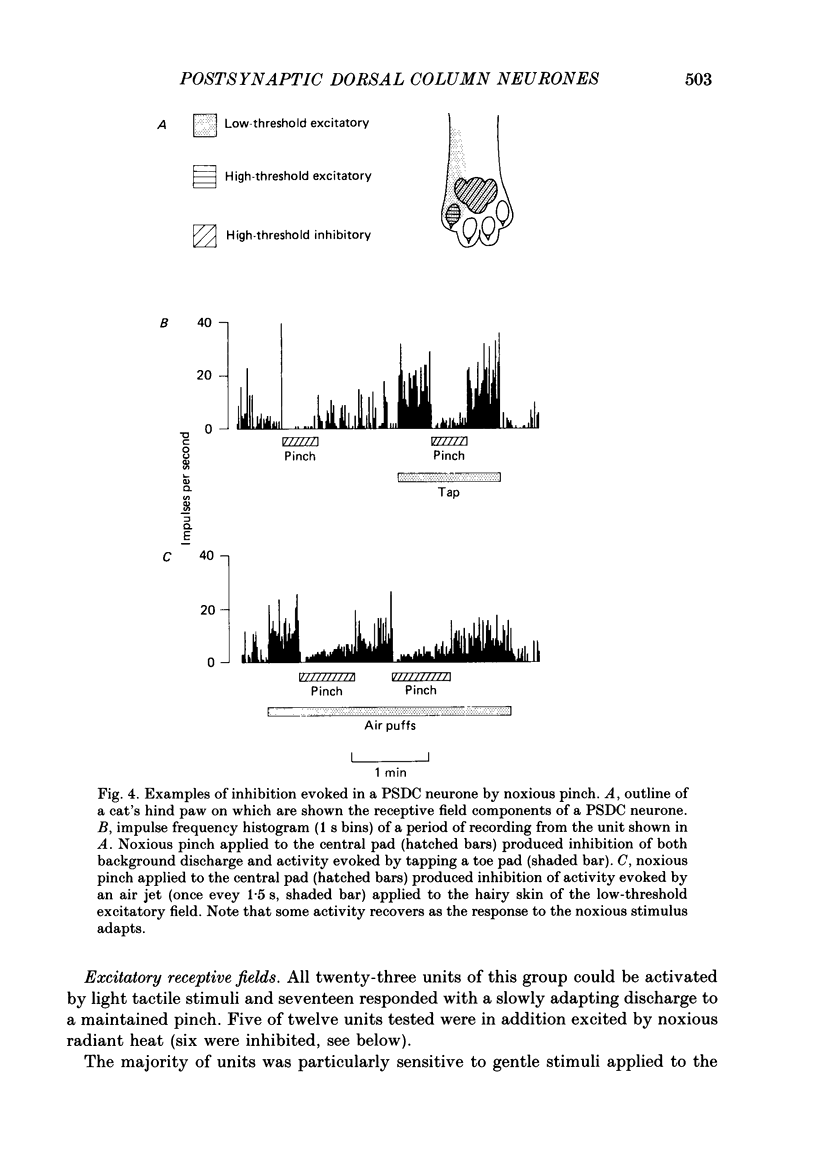
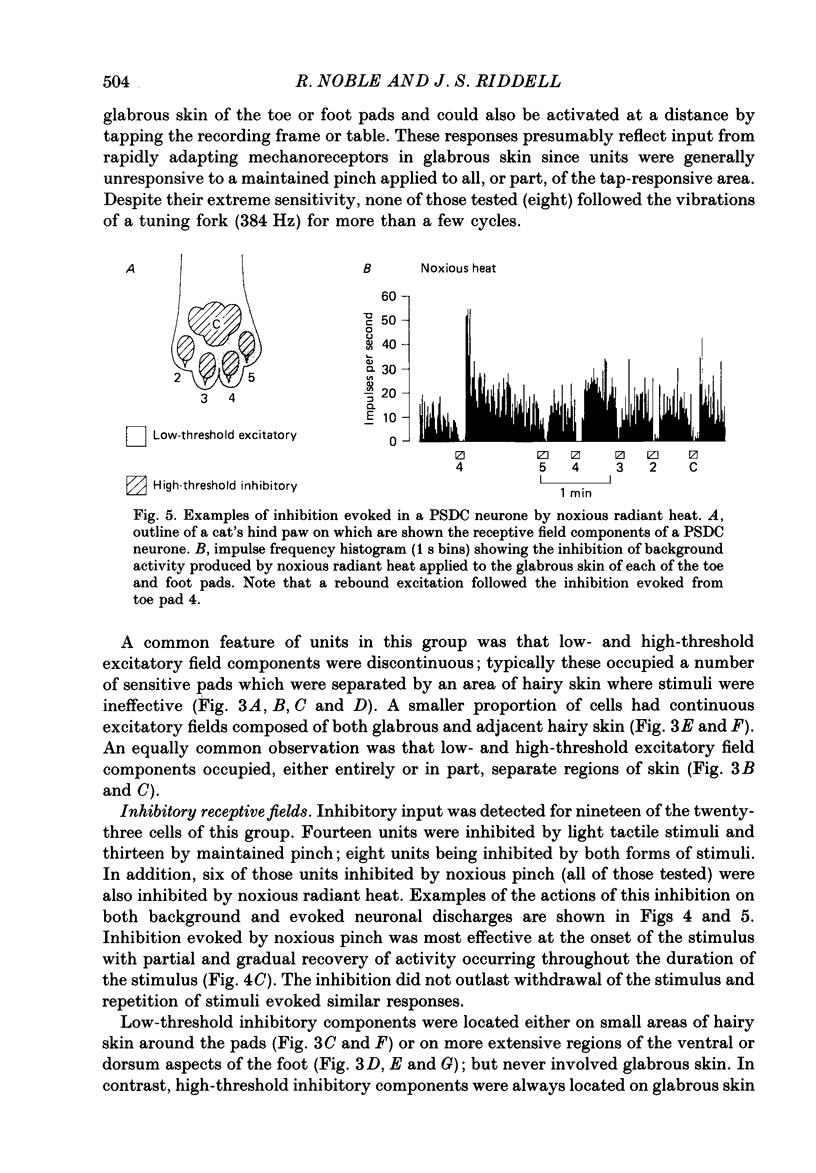
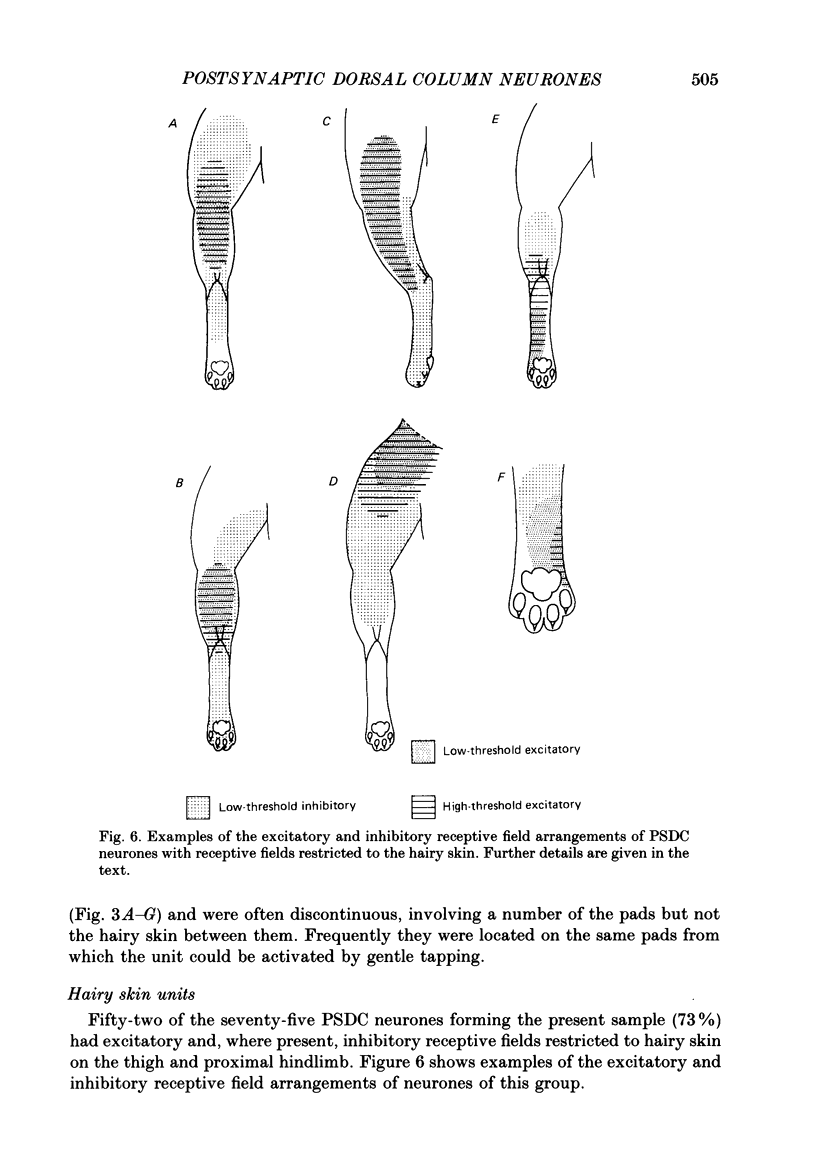
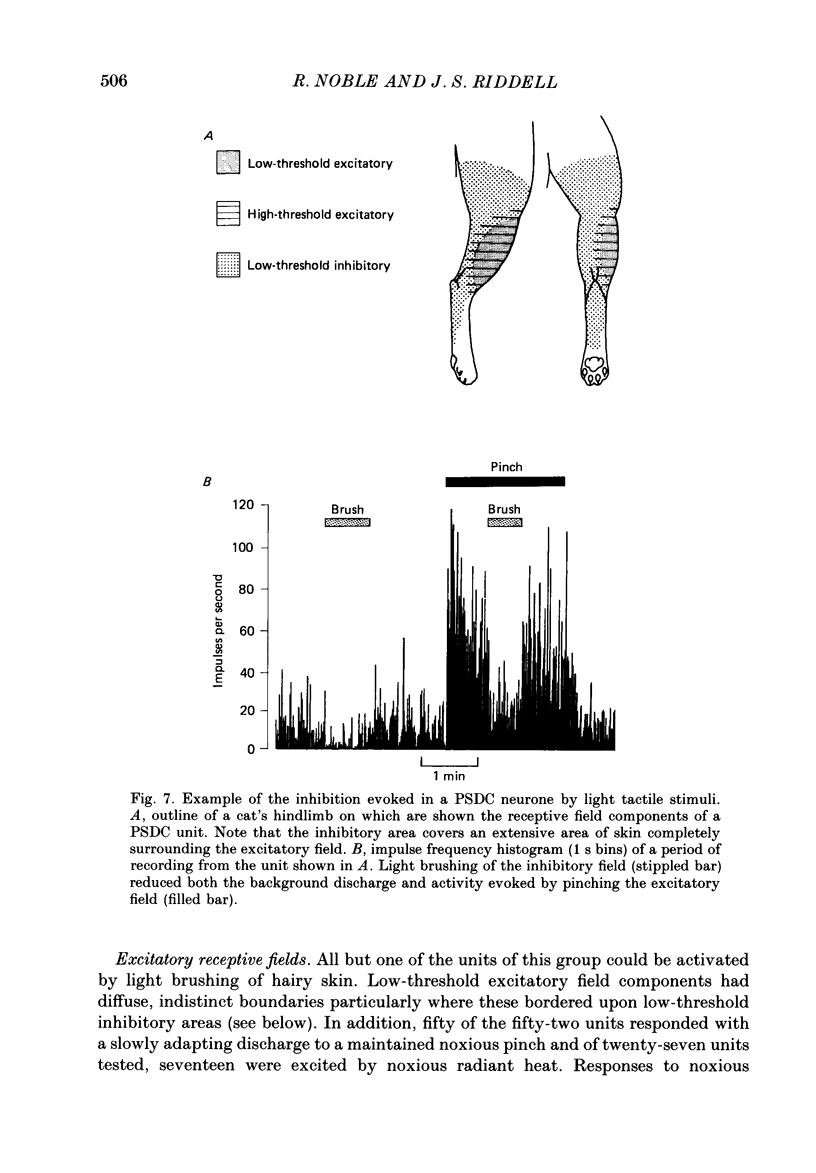






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSSON S. A. Projection of different spinal pathways to the second somatic sensory area in cat. Acta Physiol Scand Suppl. 1962;56(194):1–74. [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Aoki M. Afferent inhibition on various types of cats cuneate neurons induced by dynamic and steady tactile stimuli. Brain Res. 1981 Sep 28;221(2):257–269. doi: 10.1016/0006-8993(81)90776-9. [DOI] [PubMed] [Google Scholar]
- Baker M. A. Spontaneous and evoked activity of neurones in the somatosensory thalamus of the waking cat. J Physiol. 1971 Sep;217(2):359–379. doi: 10.1113/jphysiol.1971.sp009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. A., Tyner C. F., Towe A. L. Observations on single neurons recorded in the sigmoid gyri of awake, nonparalyzed cats. Exp Neurol. 1971 Sep;32(3):388–403. doi: 10.1016/0014-4886(71)90006-9. [DOI] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Nishikawa N., Lu G. W., Hoffert M. J., Dubner R. The morphology of dorsal column postsynaptic spinomedullary neurons in the cat. J Comp Neurol. 1984 Apr 20;224(4):568–578. doi: 10.1002/cne.902240406. [DOI] [PubMed] [Google Scholar]
- Brenowitz G. L., Pubols L. M. Increased response to sural nerve input in the dorsal horn following chronic spinal cord hemisection. Brain Res. 1981 Mar 16;208(2):421–425. doi: 10.1016/0006-8993(81)90570-9. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol. 1983 Apr;337:575–588. doi: 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. An intracellular study of spinocervical tract cell responses to natural stimuli and single hair afferent fibres in cats. J Physiol. 1987 Jan;382:331–354. doi: 10.1113/jphysiol.1987.sp016370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R., Riddell J. S. Relations between spinocervical and post-synaptic dorsal column neurones in the cat. J Physiol. 1986 Dec;381:333–349. doi: 10.1113/jphysiol.1986.sp016330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones revealed by intracellular injection of horseradish peroxidase. J Physiol. 1977 Sep;270(3):747–764. doi: 10.1113/jphysiol.1977.sp011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrzycka E., NAil B. S., Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive "tactile" neurones. J Physiol. 1977 Jun;268(1):251–270. doi: 10.1113/jphysiol.1977.sp011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart A. M. Cells of the dorsal column nuclei projecting down into the spinal cord. J Physiol. 1971 Dec;219(2):29P–30P. [PubMed] [Google Scholar]
- Devor M., Wall P. D. Dorsal horn cells with proximal cutaneous receptive fields. Brain Res. 1976 Dec 17;118(2):325–328. doi: 10.1016/0006-8993(76)90719-8. [DOI] [PubMed] [Google Scholar]
- Enevoldson T. P., Gordon G. Spinally projecting neurons in the dorsal column nuclei: distribution, dendritic trees and axonal projections. A retrograde HRP study in the cat. Exp Brain Res. 1984;54(3):538–550. doi: 10.1007/BF00235479. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. J Physiol. 1977 Feb;265(2):549–563. doi: 10.1113/jphysiol.1977.sp011730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., PAINE C. H. Functional organization in nucleus gracilis of the cat. J Physiol. 1960 Sep;153:331–349. doi: 10.1113/jphysiol.1960.sp006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. P. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976 Jan;39(1):71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- Gerhart K. D., Wilcox T. K., Chung J. M., Willis W. D. Inhibition of nociceptive and nonnociceptive responses of primate spinothalamic cells by stimulation in medial brain stem. J Neurophysiol. 1981 Jan;45(1):121–136. doi: 10.1152/jn.1981.45.1.121. [DOI] [PubMed] [Google Scholar]
- Gobel S., Falls W. M., Humphrey E. Morphology and synaptic connections of ultrafine primary axons in lamina I of the spinal dorsal horn: candidates for the terminal axonal arbors of primary neurons with unmyelinated (C) axons. J Neurosci. 1981 Oct;1(10):1163–1179. doi: 10.1523/JNEUROSCI.01-10-01163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G., Manson J. R. Cutaneous receptive fields of single nerve cells in the thalamus of the cat. Nature. 1967 Aug 5;215(5101):597–599. doi: 10.1038/215597a0. [DOI] [PubMed] [Google Scholar]
- Hillman P., Wall P. D. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res. 1969;9(4):284–306. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Spencer W. A., Younkin S. G. Spatial and temporal features of afferent inhibition of thalamocortical relay cells. J Neurophysiol. 1979 Sep;42(5):1450–1460. doi: 10.1152/jn.1979.42.5.1450. [DOI] [PubMed] [Google Scholar]
- Kamogawa H., Bennett G. J. Dorsal column postsynaptic neurons in the cat are excited by myelinated nociceptors. Brain Res. 1986 Feb 5;364(2):386–390. doi: 10.1016/0006-8993(86)90853-x. [DOI] [PubMed] [Google Scholar]
- Knyihar-Csillik E., Csillik B., Rakic P. Ultrastructure of normal and degenerating glomerular terminals of dorsal root axons in the substantia gelatinosa of the rhesus monkey. J Comp Neurol. 1982 Oct 1;210(4):357–375. doi: 10.1002/cne.902100404. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. J Neurophysiol. 1977 Nov;40(6):1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- Laskin S. E., Spencer W. A. Cutaneous masking. II. Geometry of excitatory andinhibitory receptive fields of single units in somatosensory cortex of the cat. J Neurophysiol. 1979 Jul;42(4):1061–1082. doi: 10.1152/jn.1979.42.4.1061. [DOI] [PubMed] [Google Scholar]
- Lu G. W., Bennett G. J., Nishikawa N., Hoffert M. J., Dubner R. Extra- and intracellular recordings from dorsal column postsynaptic spinomedullary neurons in the cat. Exp Neurol. 1983 Nov;82(2):456–477. doi: 10.1016/0014-4886(83)90417-x. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POWELL T. P. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp. 1959 Oct;105:201–232. [PubMed] [Google Scholar]
- Maxwell D. J., Fyffe R. E., Brown A. G. Fine structure of normal and degenerating primary afferent boutons associated with characterized spinocervical tract neurons in the cat. Neuroscience. 1984 May;12(1):151–163. doi: 10.1016/0306-4522(84)90144-1. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Fyffe R. E., Brown A. G. Fine structure of spinocervical tract neurones and the synaptic boutons in contact with them. Brain Res. 1982 Feb 11;233(2):394–399. doi: 10.1016/0006-8993(82)91212-4. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Koerber H. R., Bannatyne B. A. Light and electron microscopy of contacts between primary afferent fibres and neurones with axons ascending the dorsal columns of the feline spinal cord. Neuroscience. 1985 Oct;16(2):375–394. doi: 10.1016/0306-4522(85)90010-7. [DOI] [PubMed] [Google Scholar]
- POGGIO G. F., MOUNTCASTLE V. B. THE FUNCTIONAL PROPERTIES OF VENTROBASAL THALAMIC NEURONSSTUDIED IN UNANESTHETIZED MONKEYS. J Neurophysiol. 1963 Sep;26:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- Price D. D., Hayes R. L., Ruda M., Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol. 1978 Jul;41(4):933–947. doi: 10.1152/jn.1978.41.4.933. [DOI] [PubMed] [Google Scholar]
- Pubols L. M., Goldberger M. E. Recovery of function in dorsal horn following partial deafferentation. J Neurophysiol. 1980 Jan;43(1):102–117. doi: 10.1152/jn.1980.43.1.102. [DOI] [PubMed] [Google Scholar]
- Réthelyi M., Light A. R., Perl E. R. Synaptic complexes formed by functionally defined primary afferent units with fine myelinated fibers. J Comp Neurol. 1982 Jun 1;207(4):381–393. doi: 10.1002/cne.902070409. [DOI] [PubMed] [Google Scholar]
- TAUB A., BISHOP P. O. THE SPINOCERVICAL TRACT: DORSAL COLUMN LINKAGE, CONDUCTION VELOCITY, PRIMARY AFFERENT SPECTRUM. Exp Neurol. 1965 Sep;13:1–21. doi: 10.1016/0014-4886(65)90002-6. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis W. D., Trevino D. L., Coulter J. D., Maunz R. A. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol. 1974 Mar;37(2):358–372. doi: 10.1152/jn.1974.37.2.358. [DOI] [PubMed] [Google Scholar]


