Abstract
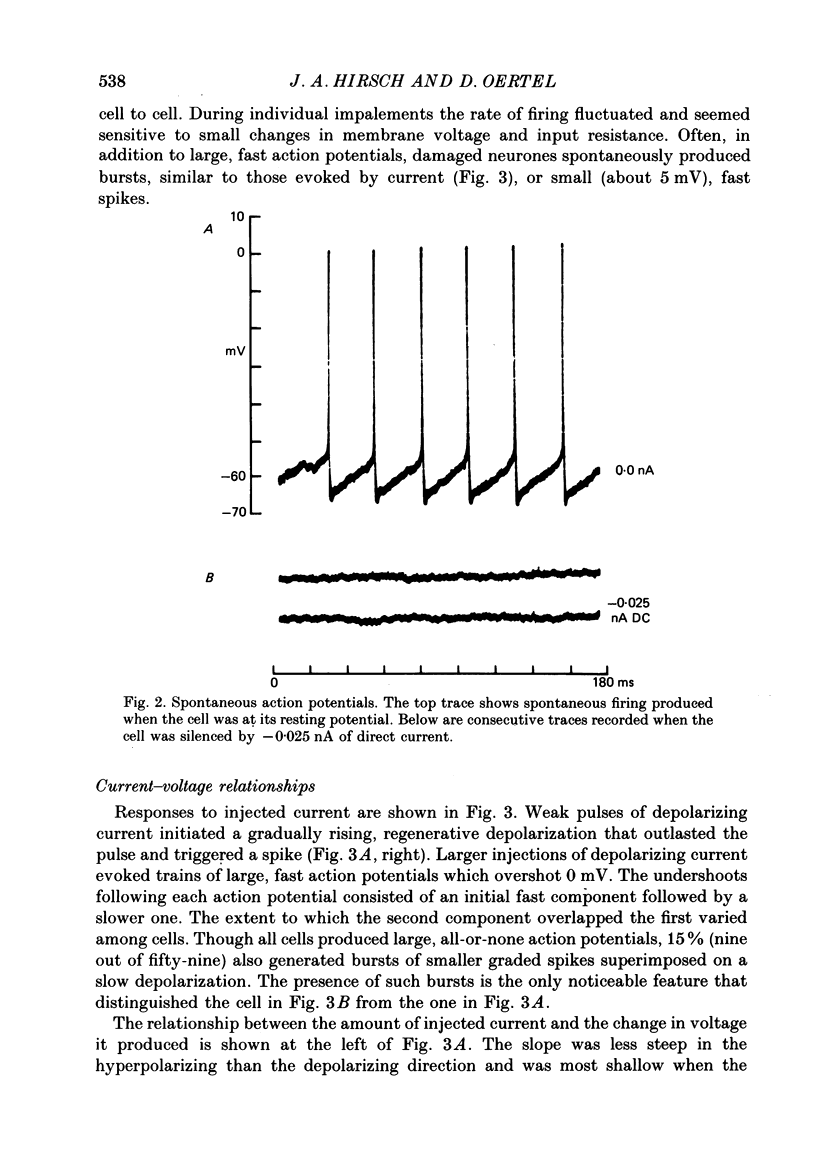
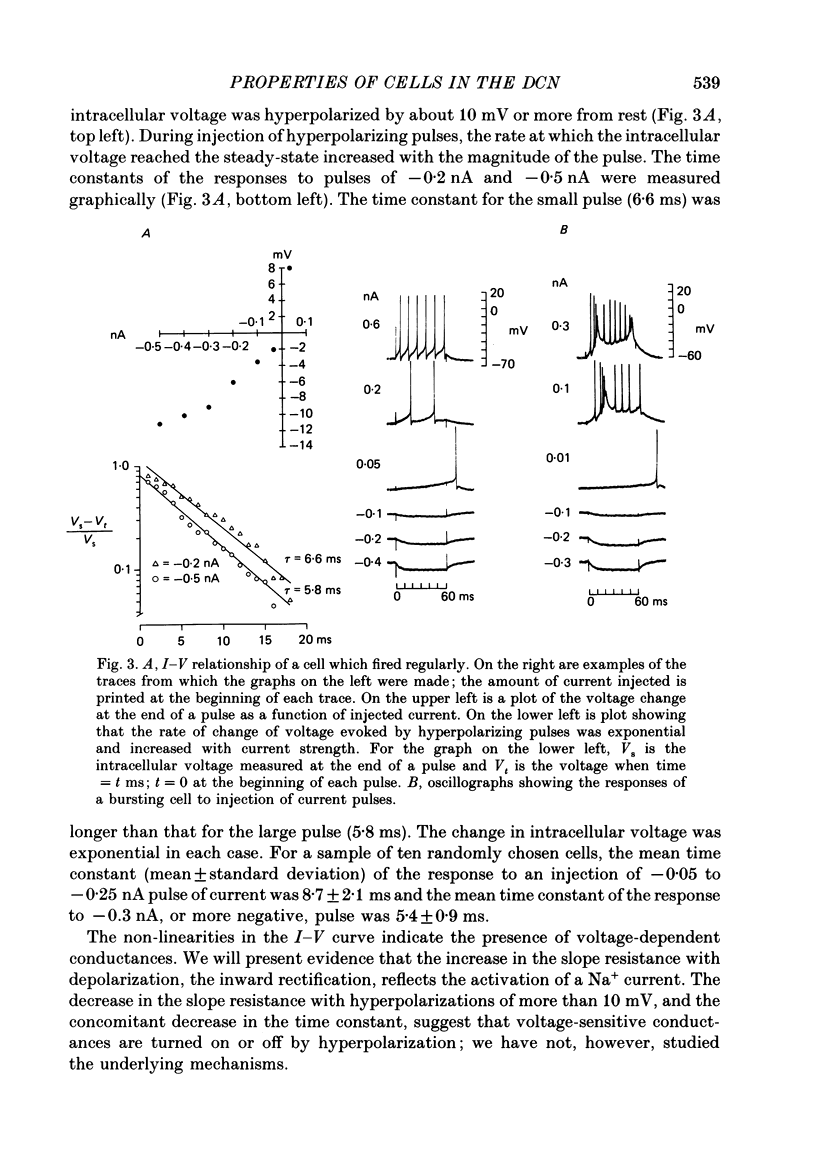
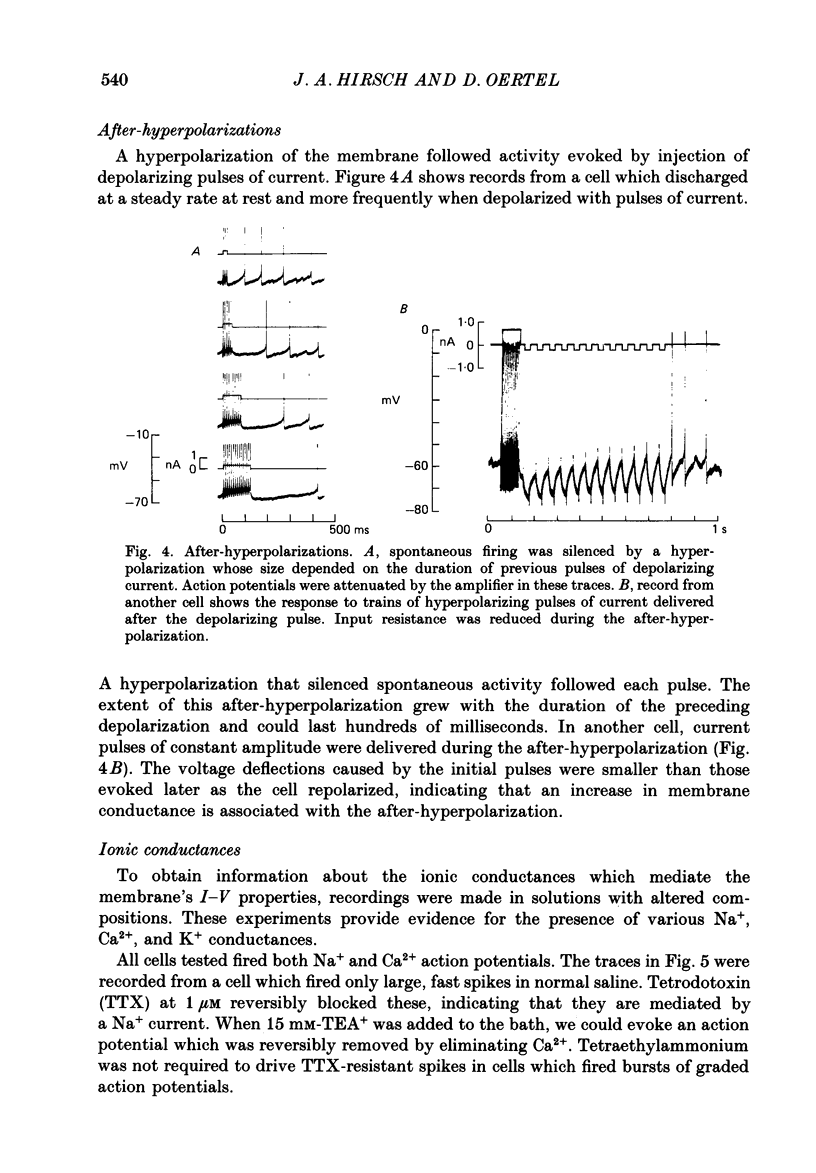
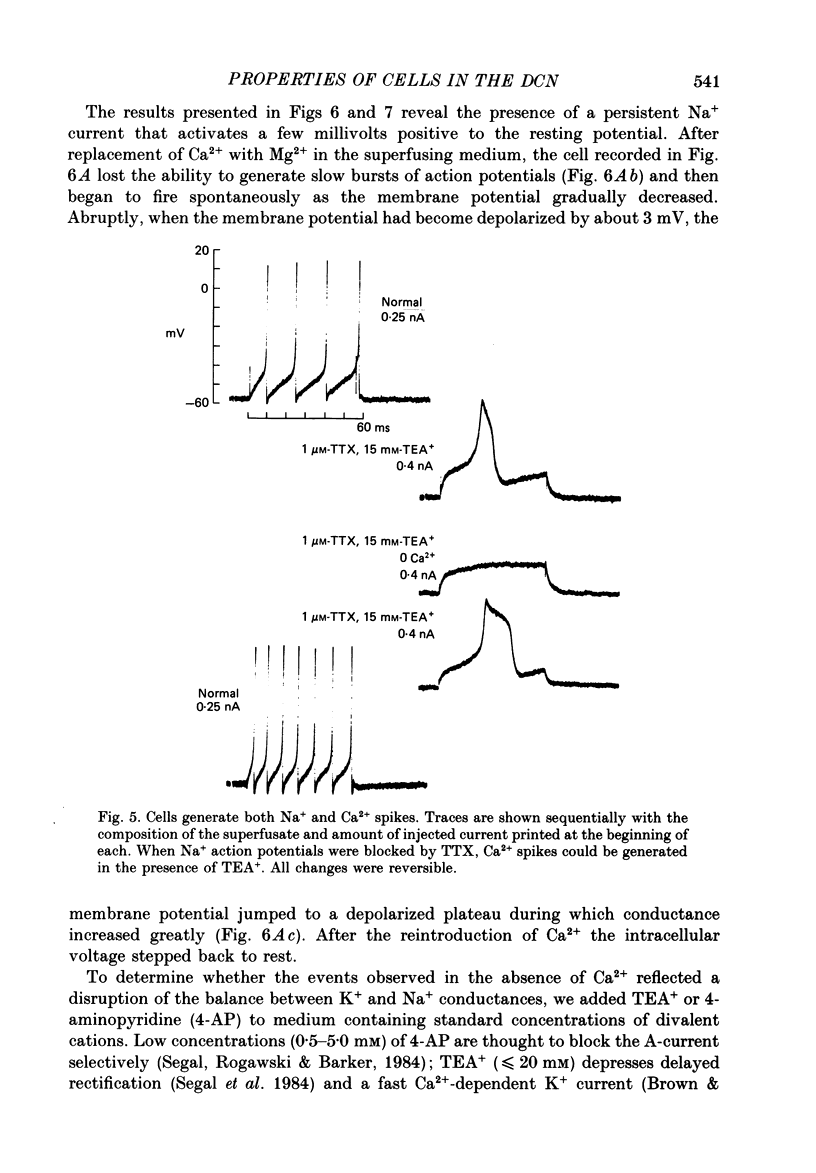
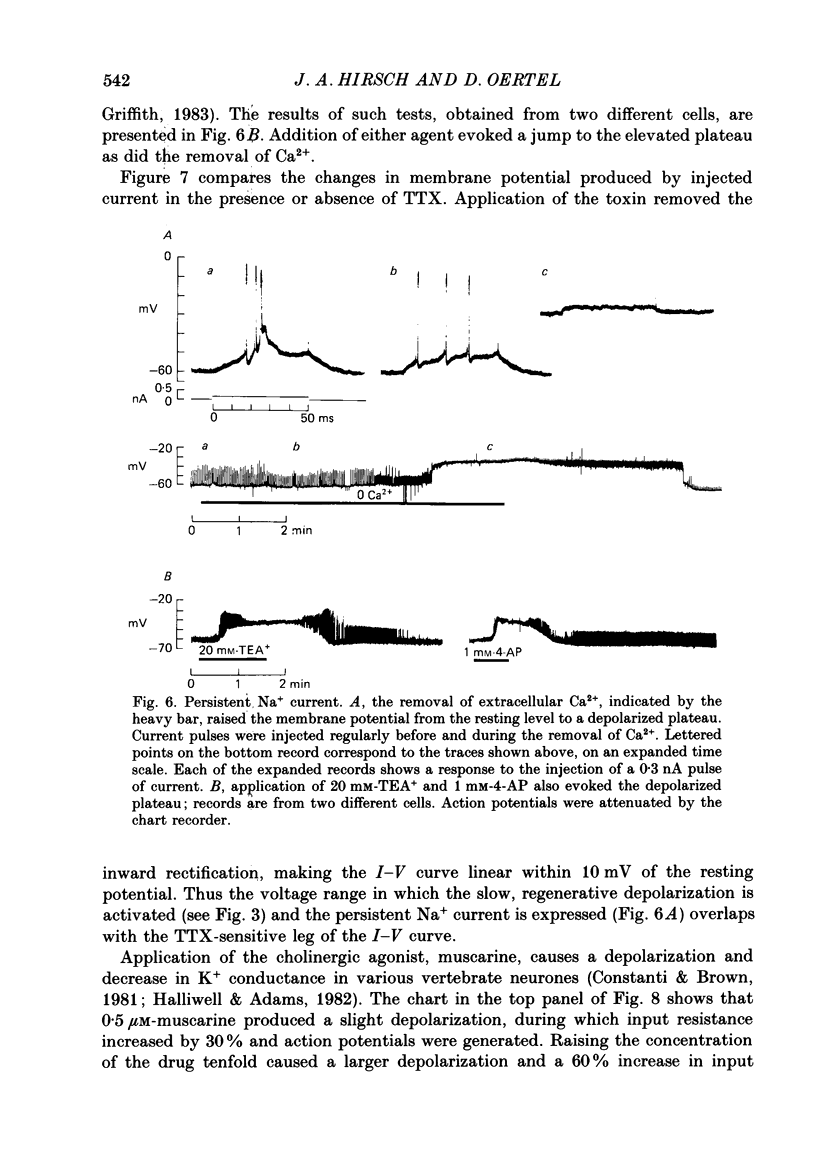
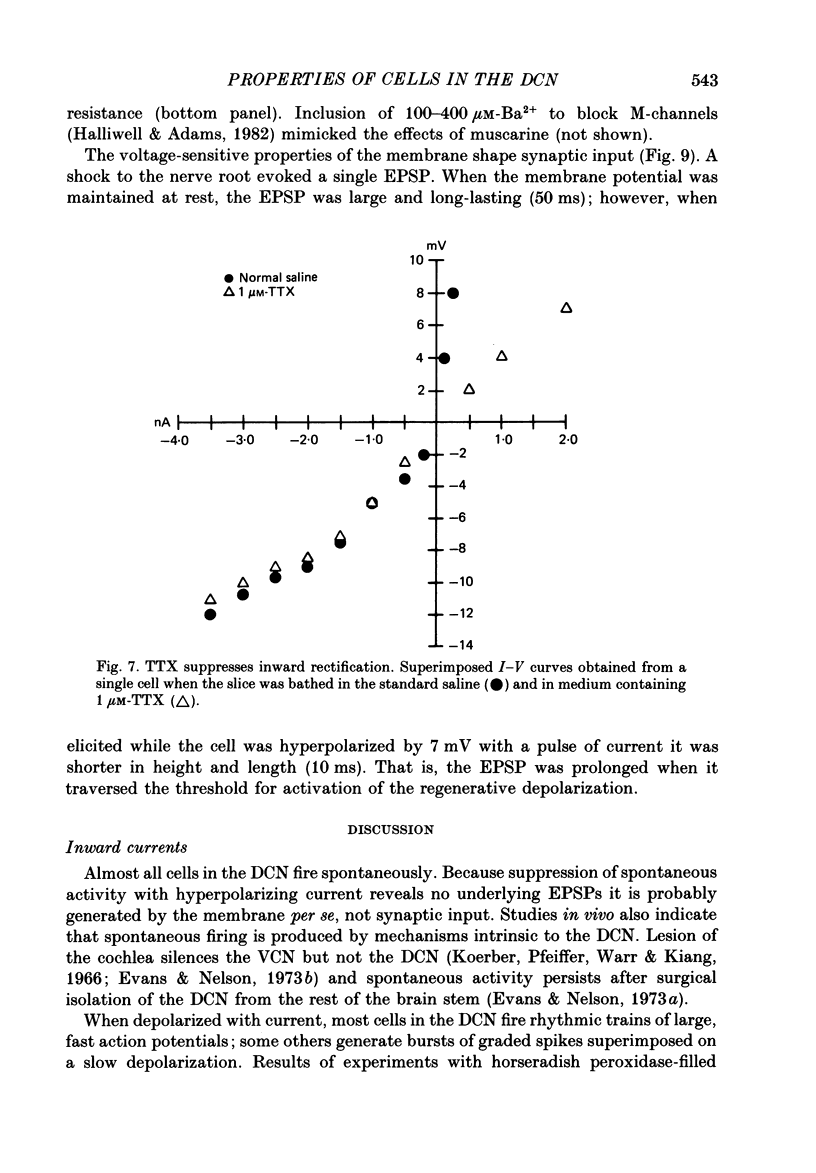
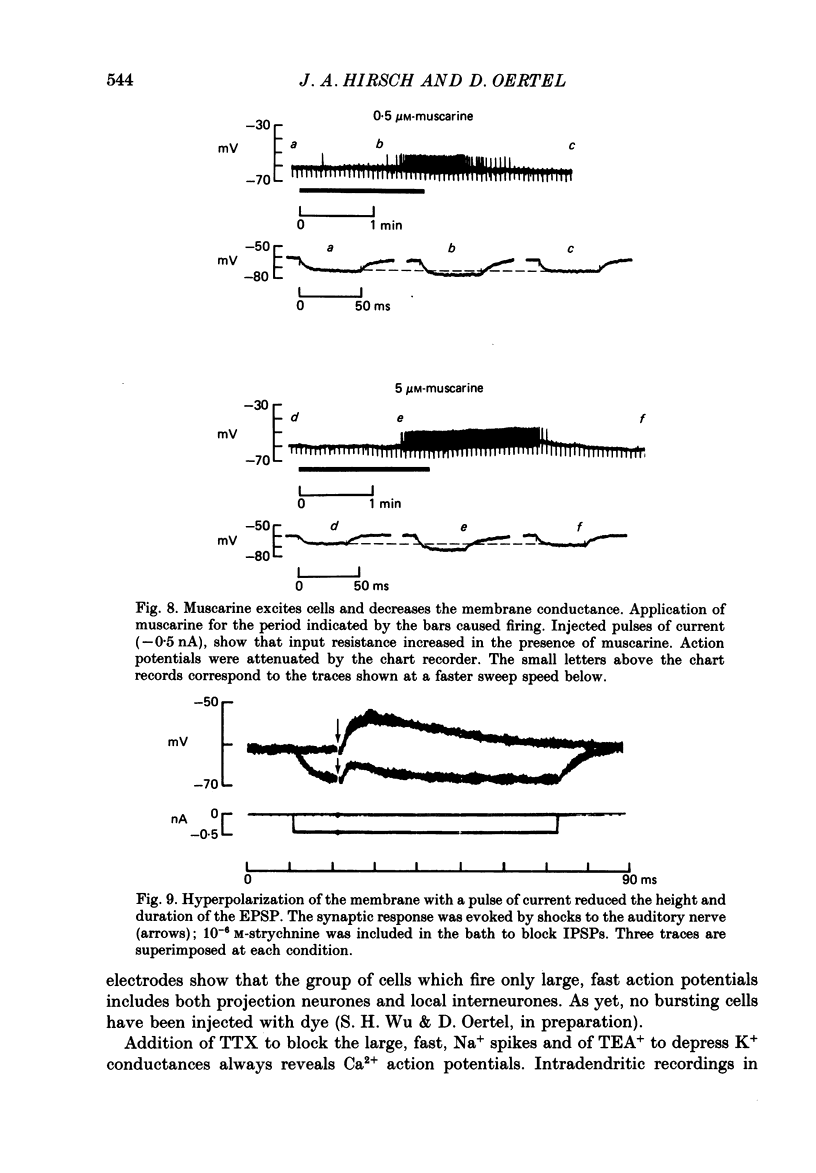
1. Intracellular recordings were made from the dorsal cochlear nucleus (DCN) in slices of the cochlear nuclear complex. Probably the larger and most frequent cells were impaled. 2. The steady-state current-voltage (I-V) properties of all cells impaled were nonlinear. The I-V curve was steepest in the voltage range depolarized from the resting potential and most shallow when the cell was hyperpolarized from rest by more than about 10 mV. Thus, the inwardly rectifying I-V characteristics of cells in the DCN distinguish them from those of ventral cochlear nuclear neurones (Oertel, 1983). 3. When depolarized with current, most cells fired trains of large, all-or-none action potentials. The undershoot after single spikes comprised an initial, fast component followed by a second, slower wave. A few cells (15%) generated bursts of smaller, graded spikes in addition to the large ones. 4. Repetitive firing evoked by depolarizing pulses of current was followed by an after-hyperpolarization whose magnitude depended on the strength and duration of the preceding current pulse. 5. Blocking the large action potentials with tetrodotoxin (TTX) revealed Ca2+-dependent spikes in all cells examined. 6. The steady-state I-V relationship became linear in the presence of TTX, suggesting that a persistent Na+ conductance probably mediates the inward rectification seen above the resting potential. 7. Muscarine at micromolar concentrations excited cells and increased their input resistance.
Full text
PDF
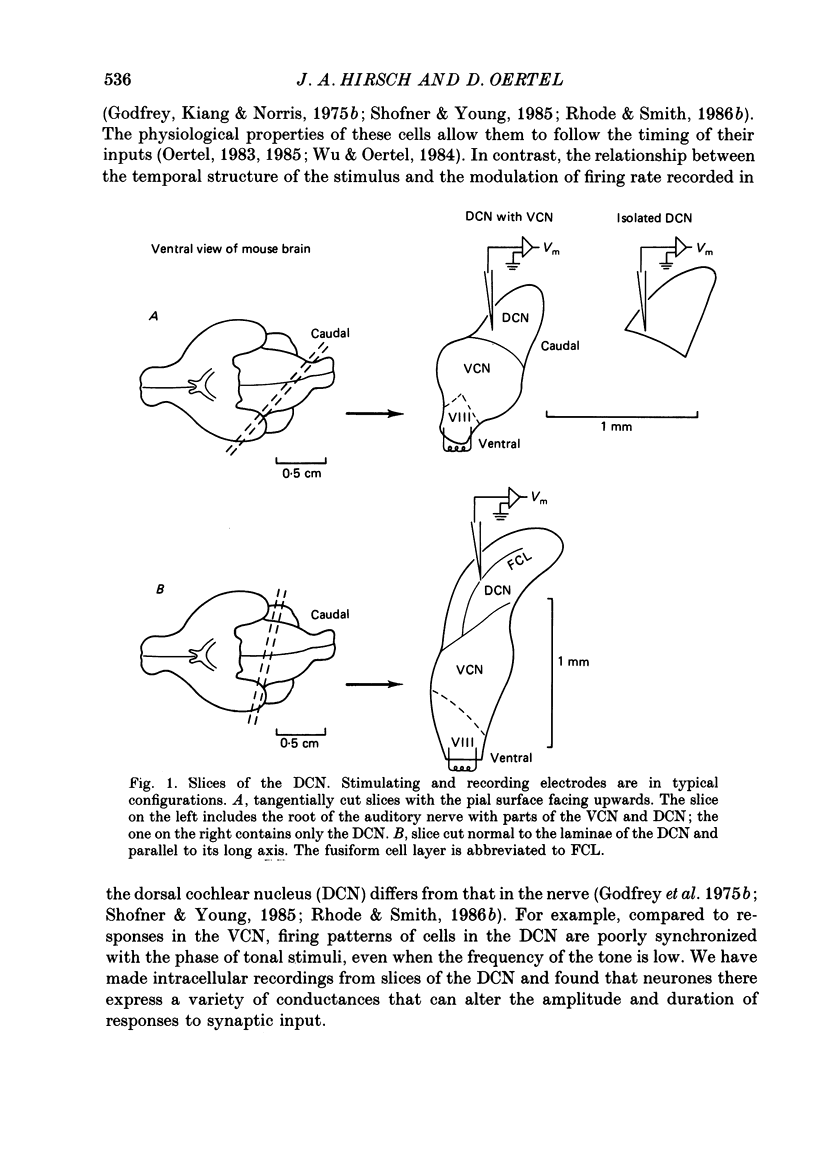





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo L. S., Masukawa L. M., Prince D. A. Electrophysiology of isolated hippocampal pyramidal dendrites. J Neurosci. 1982 Nov;2(11):1614–1622. doi: 10.1523/JNEUROSCI.02-11-01614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comis S. D., Davies W. E. Acetylcholine as a transmitter in the cat auditory system. J Neurochem. 1969 Mar;16(3):423–429. doi: 10.1111/j.1471-4159.1969.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Comis S. D., Whitfield I. C. Influence of centrifugal pathways on unit activity in the cochlear nucleus. J Neurophysiol. 1968 Jan;31(1):62–68. doi: 10.1152/jn.1968.31.1.62. [DOI] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Evans E. F., Nelson P. G. On the functional relationship between the dorsal and ventral divisions of the cochlear nucleus of the cat. Exp Brain Res. 1973 Jun 29;17(4):428–442. doi: 10.1007/BF00234104. [DOI] [PubMed] [Google Scholar]
- Evans E. F., Nelson P. G. The responses of single neurones in the cochlear nucleus of the cat as a function of their location and the anaesthetic state. Exp Brain Res. 1973 Jun 29;17(4):402–427. doi: 10.1007/BF00234103. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Seroogy K. B. Visual and auditory pathways contain cholecystokinin: evidence from immunofluorescence and retrograde tracing. Neurosci Lett. 1984 Mar 9;45(1):81–87. doi: 10.1016/0304-3940(84)90333-1. [DOI] [PubMed] [Google Scholar]
- Fekete D. M., Rouiller E. M., Liberman M. C., Ryugo D. K. The central projections of intracellularly labeled auditory nerve fibers in cats. J Comp Neurol. 1984 Nov 1;229(3):432–450. doi: 10.1002/cne.902290311. [DOI] [PubMed] [Google Scholar]
- Frostholm A., Rotter A. Autoradiographic localization of receptors in the cochlear nucleus of the mouse. Brain Res Bull. 1986 Feb;16(2):189–203. doi: 10.1016/0361-9230(86)90033-x. [DOI] [PubMed] [Google Scholar]
- Gerstein G. L., Butler R. A., Erulkar S. D. Excitation and inhibition in cochlear nucleus. I. Tone-burst stimulation. J Neurophysiol. 1968 Jul;31(4):526–536. doi: 10.1152/jn.1968.31.4.526. [DOI] [PubMed] [Google Scholar]
- Godfrey D. A., Kiang N. Y., Norris B. E. Single unit activity in the dorsal cochlear nucleus of the cat. J Comp Neurol. 1975 Jul 15;162(2):269–284. doi: 10.1002/cne.901620207. [DOI] [PubMed] [Google Scholar]
- Godfrey D. A., Kiang N. Y., Norris B. E. Single unit activity in the posteroventral cochlear nucleus of the cat. J Comp Neurol. 1975 Jul 15;162(2):247–268. doi: 10.1002/cne.901620206. [DOI] [PubMed] [Google Scholar]
- Godfrey D. A., Matschinsky F. M. Quantitative distribution of choline acetyltransferase and acetylcholinesterase activities in the rat cochlear nucleus. J Histochem Cytochem. 1981 Jun;29(6):720–730. doi: 10.1177/29.6.7252132. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hirsch J. A., Oertel D. Synaptic connections in the dorsal cochlear nucleus of mice, in vitro. J Physiol. 1988 Feb;396:549–562. doi: 10.1113/jphysiol.1988.sp016977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber K. C., Pfeiffer R. R., Warr W. B., Kiang N. Y. Spontaneous spike discharges from single units in the cochlear nucleus after destruction of the cochlea. Exp Neurol. 1966 Oct;16(2):119–130. doi: 10.1016/0014-4886(66)90091-4. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A., Hökfelt T., Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3(10):861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Oertel D. Synaptic responses and electrical properties of cells in brain slices of the mouse anteroventral cochlear nucleus. J Neurosci. 1983 Oct;3(10):2043–2053. doi: 10.1523/JNEUROSCI.03-10-02043.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. Use of brain slices in the study of the auditory system: spatial and temporal summation of synaptic inputs in cells in the anteroventral cochlear nucleus of the mouse. J Acoust Soc Am. 1985 Jul;78(1 Pt 2):328–333. doi: 10.1121/1.392494. [DOI] [PubMed] [Google Scholar]
- Osen K. K., Roth K. Histochemical localization of cholinesterases in the cochlear nuclei of the cat, with notes on the origin of acetylcholinesterase-positive afferents and the superior olive. Brain Res. 1969 Nov;16(1):165–185. doi: 10.1016/0006-8993(69)90092-4. [DOI] [PubMed] [Google Scholar]
- Rhode W. S., Smith P. H. Encoding timing and intensity in the ventral cochlear nucleus of the cat. J Neurophysiol. 1986 Aug;56(2):261–286. doi: 10.1152/jn.1986.56.2.261. [DOI] [PubMed] [Google Scholar]
- Rhode W. S., Smith P. H., Oertel D. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol. 1983 Feb 1;213(4):426–447. doi: 10.1002/cne.902130407. [DOI] [PubMed] [Google Scholar]
- Rhode W. S., Smith P. H. Physiological studies on neurons in the dorsal cochlear nucleus of cat. J Neurophysiol. 1986 Aug;56(2):287–307. doi: 10.1152/jn.1986.56.2.287. [DOI] [PubMed] [Google Scholar]
- Segal M., Rogawski M. A., Barker J. L. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984 Feb;4(2):604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shofner W. P., Young E. D. Excitatory/inhibitory response types in the cochlear nucleus: relationships to discharge patterns and responses to electrical stimulation of the auditory nerve. J Neurophysiol. 1985 Oct;54(4):917–939. doi: 10.1152/jn.1985.54.4.917. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Starr A., Britt R. Intracellular recordings from cat cochlear nucleus during tone stimulation. J Neurophysiol. 1970 Jan;33(1):137–147. doi: 10.1152/jn.1970.33.1.137. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Spontaneous muscarinic suppression of the Ca-activated K-current in bullfrog sympathetic neurons. Brain Res. 1985 Sep 30;344(1):134–141. doi: 10.1016/0006-8993(85)91197-7. [DOI] [PubMed] [Google Scholar]
- Whipple M. R., Drescher D. G. Muscarinic receptors in the cochlear nucleus and auditory nerve of the guinea pig. J Neurochem. 1984 Jul;43(1):192–198. doi: 10.1111/j.1471-4159.1984.tb06696.x. [DOI] [PubMed] [Google Scholar]
- Wu S. H., Oertel D. Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci. 1984 Jun;4(6):1577–1588. doi: 10.1523/JNEUROSCI.04-06-01577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. D., Brownell W. E. Responses to tones and noise of single cells in dorsal cochlear nucleus of unanesthetized cats. J Neurophysiol. 1976 Mar;39(2):282–300. doi: 10.1152/jn.1976.39.2.282. [DOI] [PubMed] [Google Scholar]


