Abstract
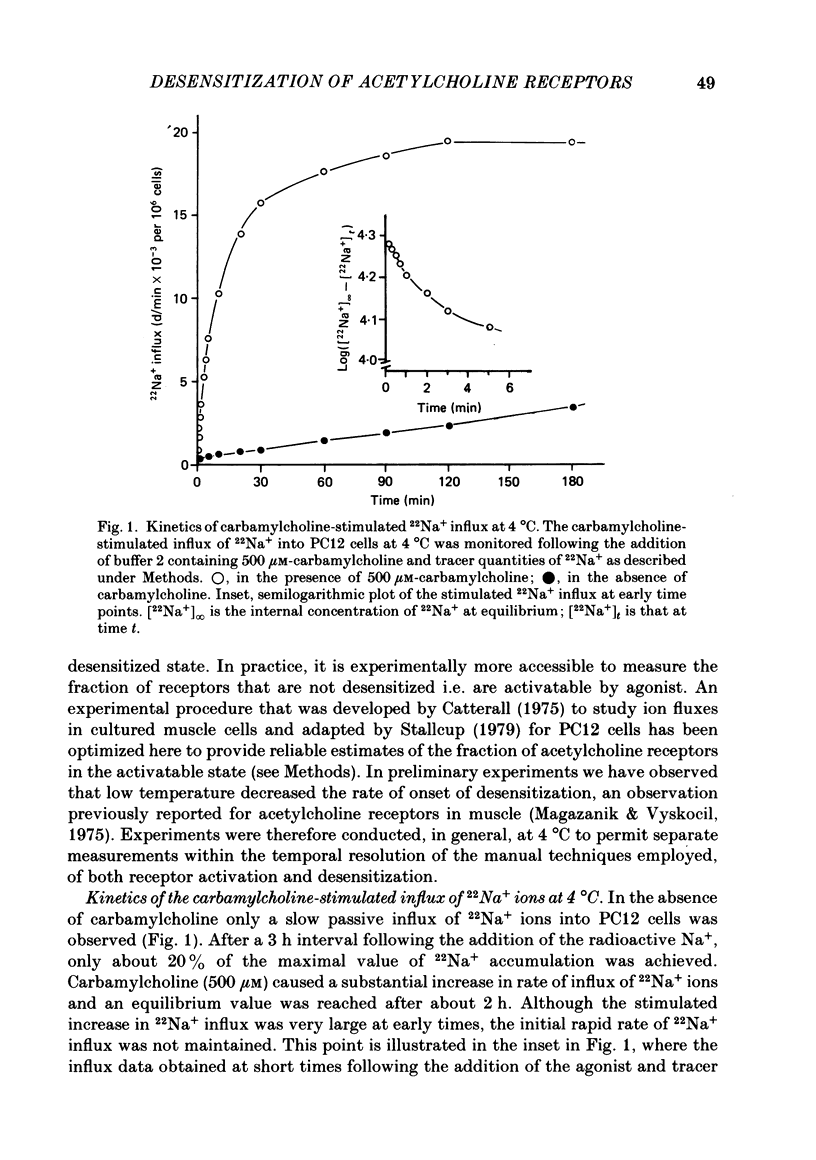
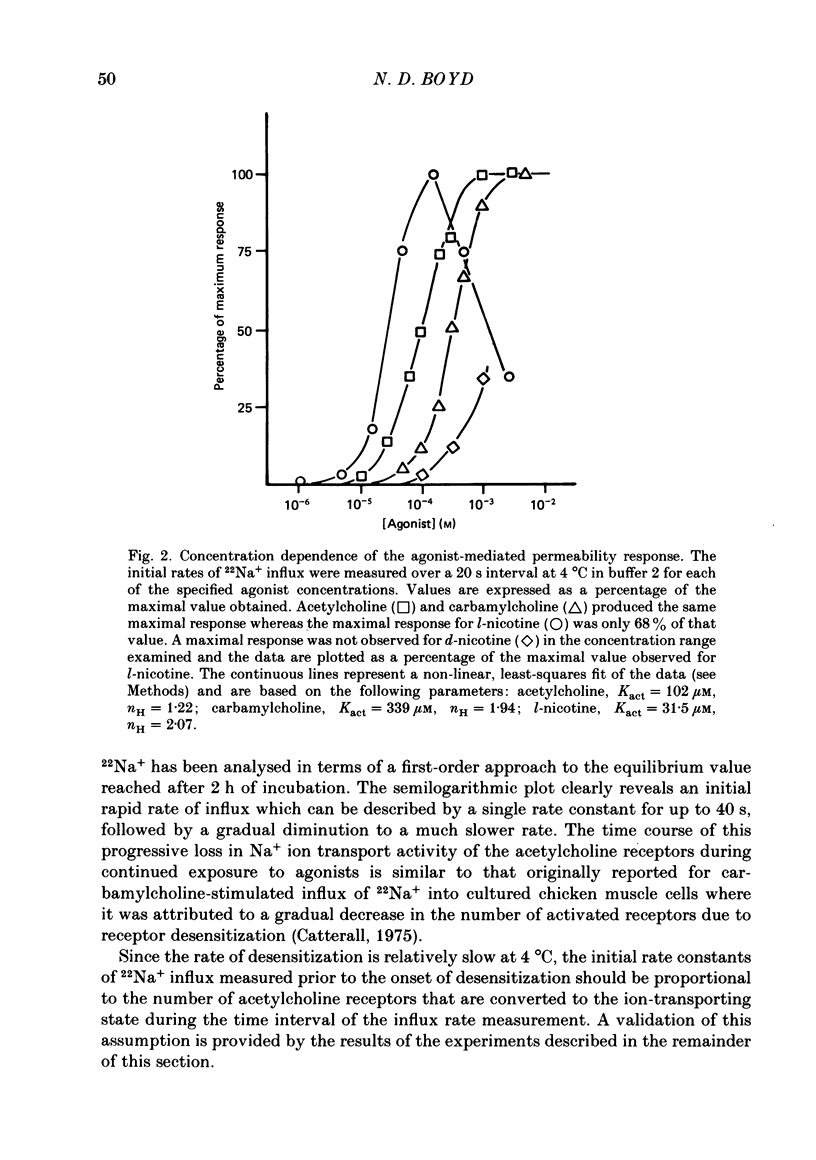
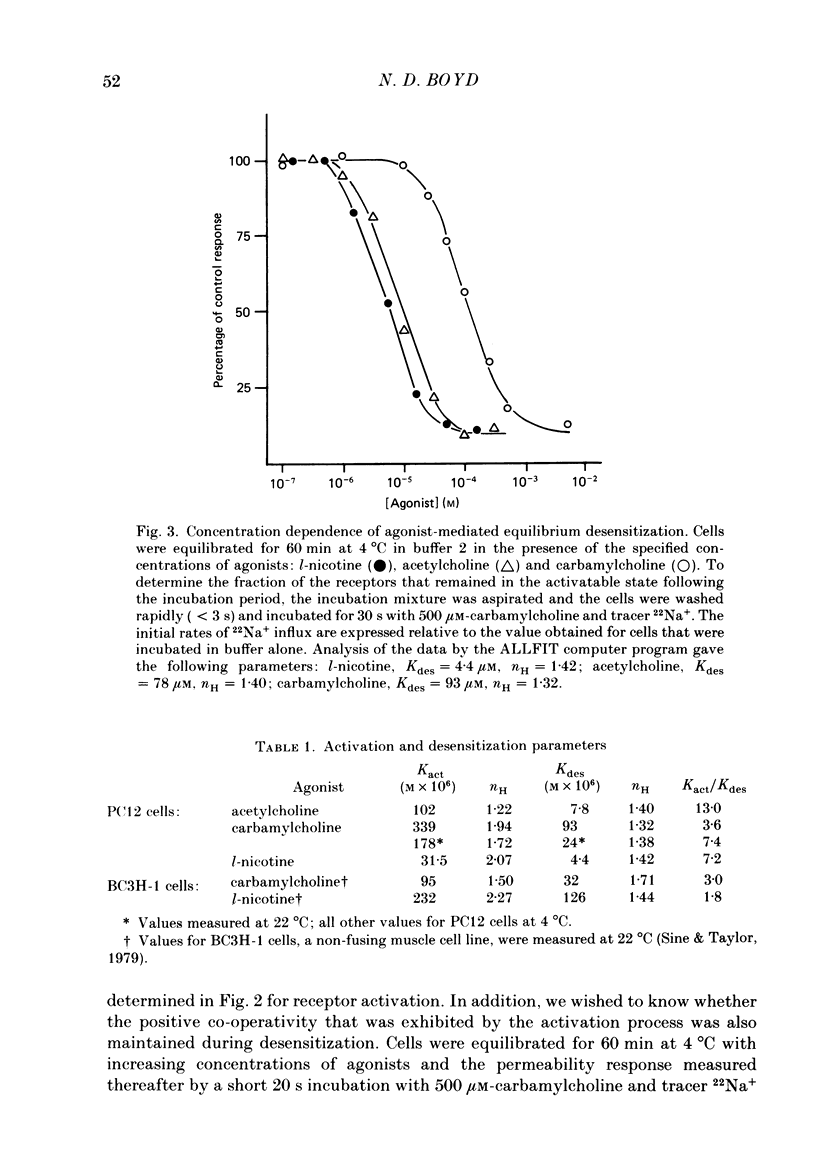
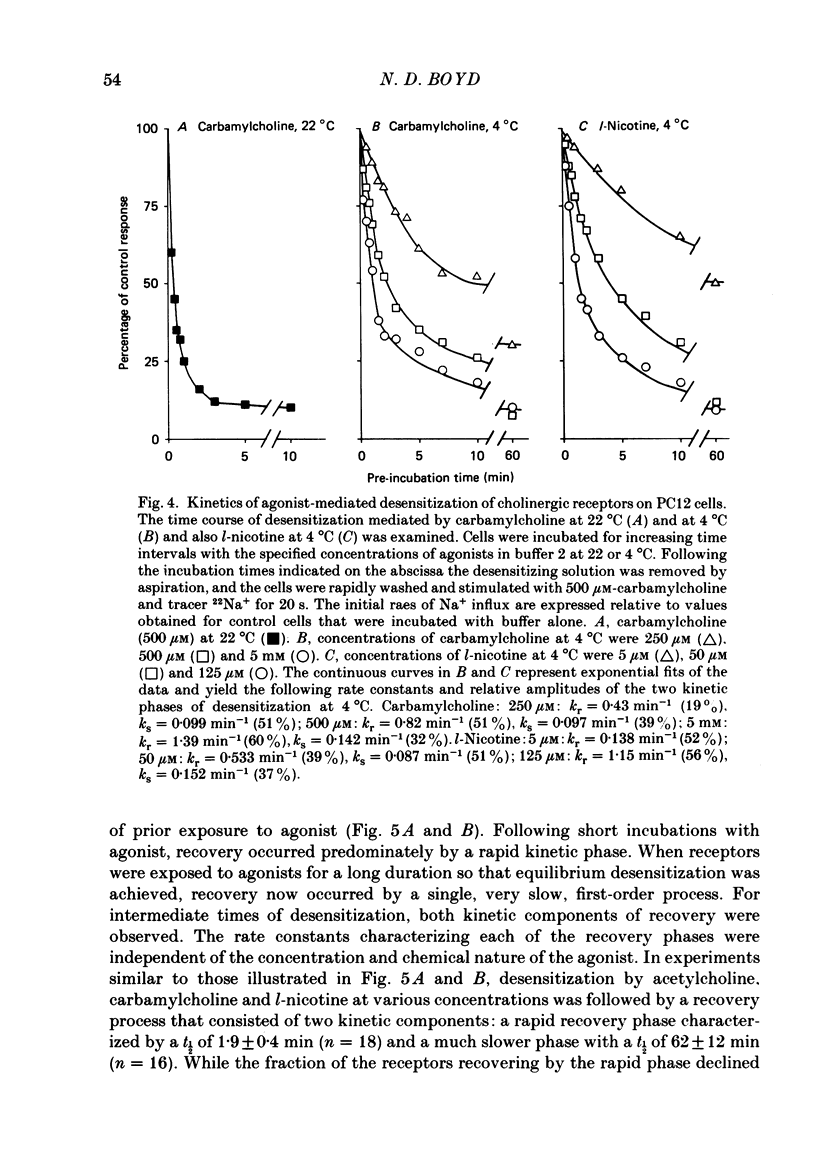
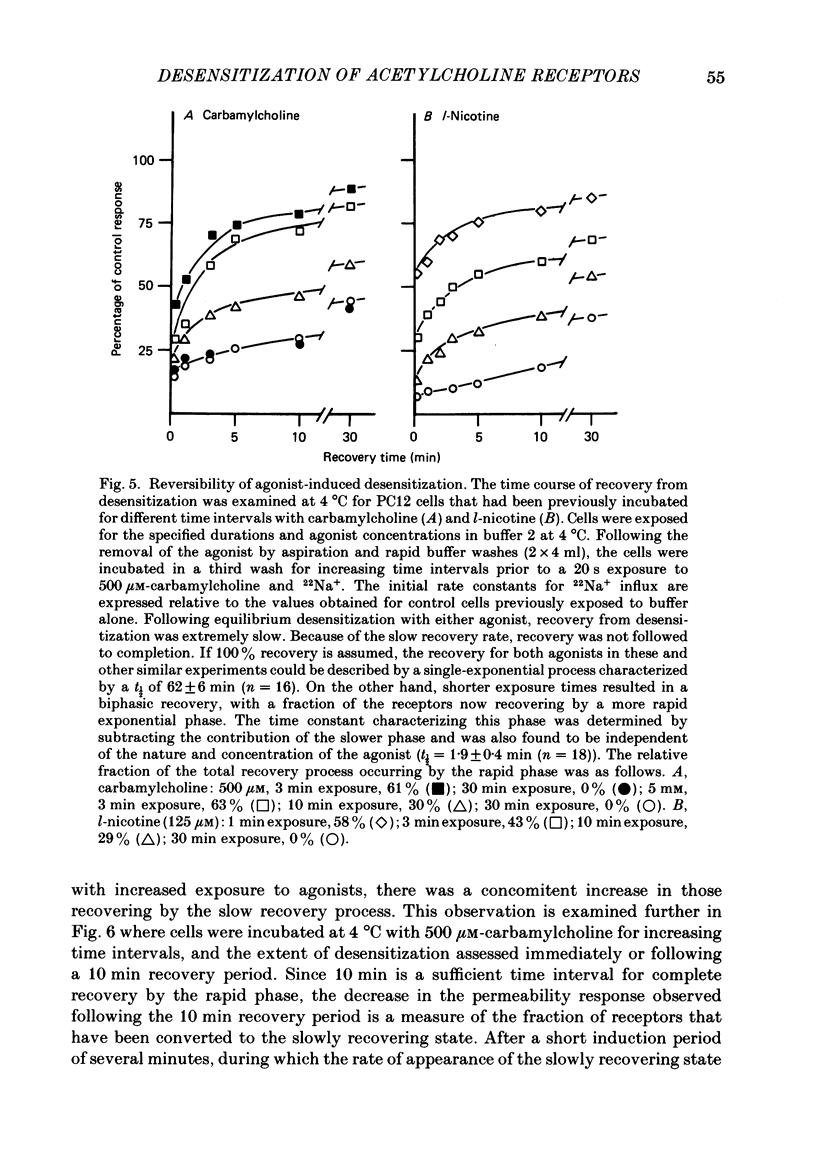
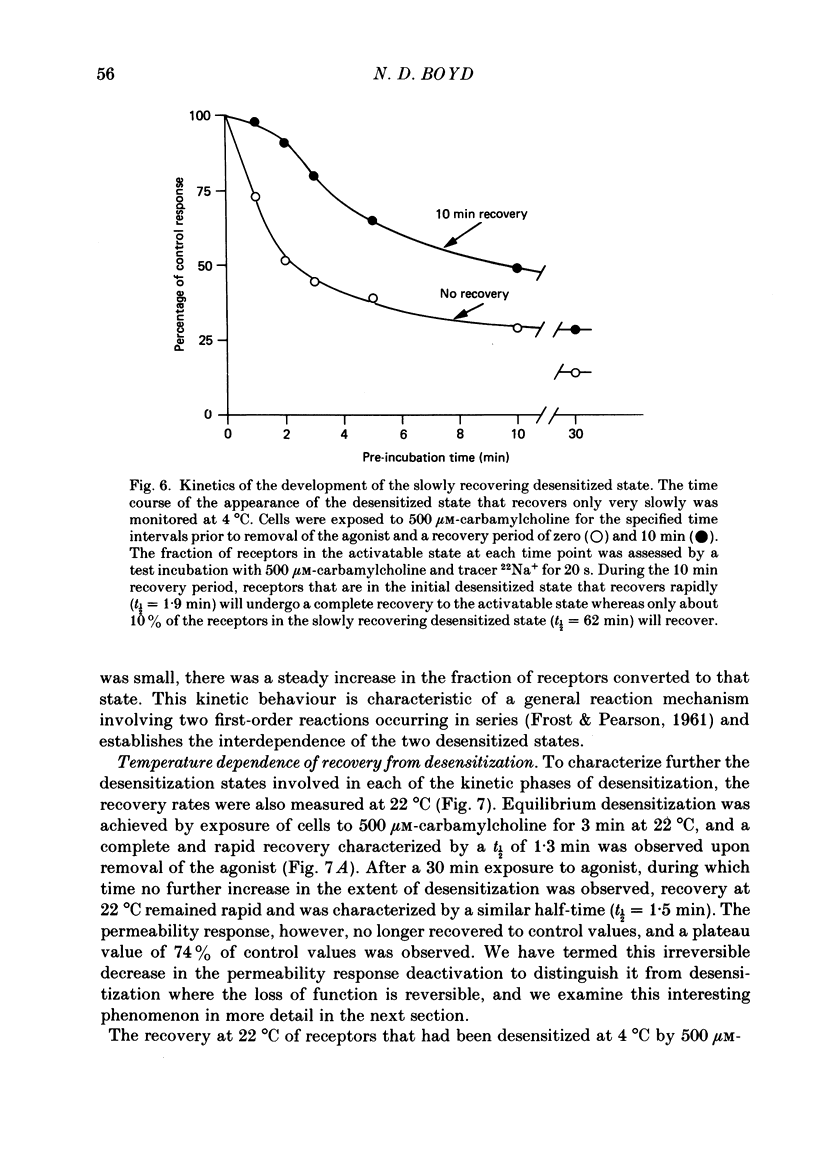
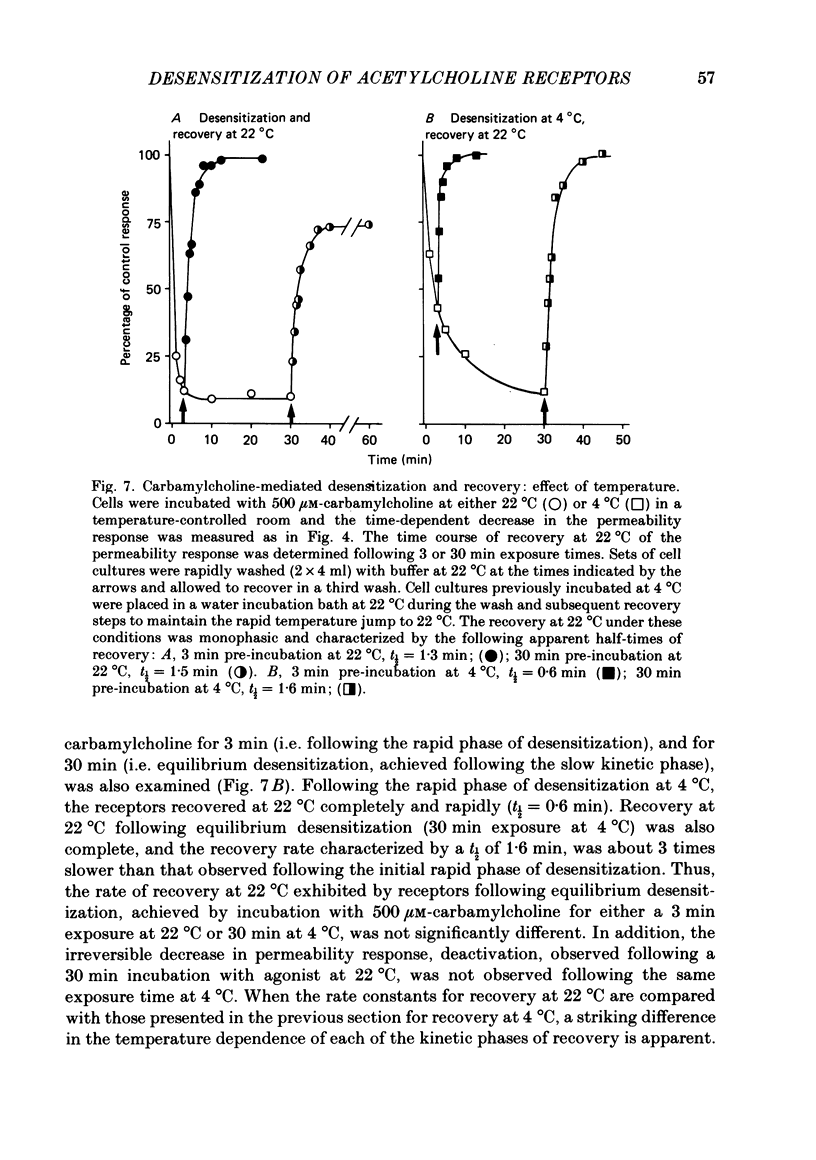
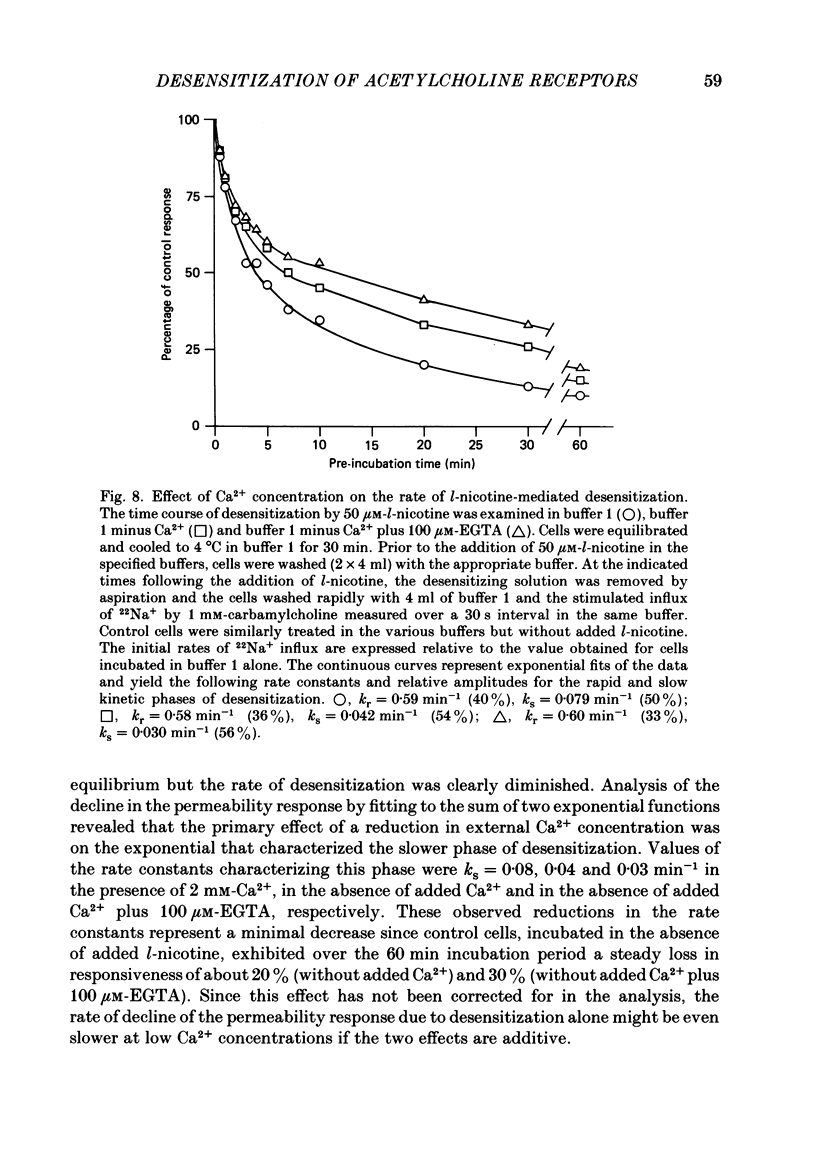
1. The desensitization of nicotinic acetylcholine receptors on the PC12 sympathetic cell line was investigated by using a 22Na+ influx assay to measure receptor activation. 2. The rate of desensitization was dependent on temperature and at 4 degrees C two distinct kinetic phases were readily discernible: a rapid phase that was characterized by rate constants that were dependent on the chemical nature and concentration of the agonist, and a slower phase that was characterized by rate constants that were less dependent on these. 3. For acetylcholine, carbamylcholine and l-nicotine, the equilibrium desensitization parameter, Kdes, the concentration that produces half-maximal desensitization, was determined and compared with the corresponding value for Kact, the concentration that results in a half-maximal increase in the permeability response. For each agonist, the value of Kdes was found to be lower than Kact, a result to be expected if desensitization is associated with a higher-affinity state of the receptor than that associated with ion channel activation. Thus, extensive receptor desensitization can occur even at agonist concentrations that do not produce appreciable channel activation. Both activation and desensitization functions exhibited positive cooperativity so that each function occurs over a narrow range of agonist concentrations. 4. Following removal of the agonist, recovery from desensitization was reversible and occurred by two distinct kinetic phases characterized by rate constants that were independent of the chemical nature and concentration of the agonist that produced the desensitization. The relative contribution of each kinetic phase of recovery was, however, dependent on the duration of prior exposure to agonist. Following short incubation periods with agonist, most of the receptors were in a rapidly recovering state. With increasing duration of exposure, progressively more of the receptors were converted to a desensitized state that recovered more slowly. 5. The rate constants associated with the two kinetic phases of recovery were dependent on the recovery temperature. Following the initial rapid phase of desensitization, recovery at 4 degrees C was characterized by a time constant, t1/2, of 1.9 min, a value that was about 3-fold greater than that observed at 22 degrees C. The rate of recovery of the desensitized state achieved following equilibrium exposures to agonists was considerably more temperature dependent: recovery of this desensitized state was characterized at 4 degrees C by a t1/2 of 62 min that was about 37-fold greater than that at 22 degrees C.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Anwyl R., Narahashi T. Desensitization of the acetylcholine receptor of denervated rat soleus muscle and the effect of calcium. Br J Pharmacol. 1980 May;69(1):91–98. doi: 10.1111/j.1476-5381.1980.tb10886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. D., Cohen J. B. Desensitization of membrane-bound Torpedo acetylcholine receptor by amine noncompetitive antagonists and aliphatic alcohols: studies of [3H]acetylcholine binding and 22Na+ ion fluxes. Biochemistry. 1984 Aug 28;23(18):4023–4033. doi: 10.1021/bi00313a003. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Cohen J. B. Kinetics of binding of [3H]acetylcholine and [3H]carbamoylcholine to Torpedo postsynaptic membranes: slow conformational transitions of the cholinergic receptor. Biochemistry. 1980 Nov 11;19(23):5344–5353. doi: 10.1021/bi00564a031. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Leeman S. E. Multiple actions of substance P that regulate the functional properties of acetylcholine receptors of clonal rat PC12 cells. J Physiol. 1987 Aug;389:69–97. doi: 10.1113/jphysiol.1987.sp016647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBassio W. A., Parsons R. L., Schnitzler R. M. Effect of ionophore X-537A on desensitization rate and tension development in potassium-depolarized muscle fibres. Br J Pharmacol. 1976 Aug;57(4):565–571. doi: 10.1111/j.1476-5381.1976.tb10386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Milfay D., Davis C. G., Diamond I. Protein phosphatase activity in acetylcholine receptor-enriched membranes. Biochem Biophys Res Commun. 1979 Apr 13;87(3):876–883. doi: 10.1016/0006-291x(79)92039-4. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Sytkowski A. J., Vogel Z., Nirenberg M. W. -Bungarotoxin used as a probe for acetylcholine receptors of cultured neurones. Nature. 1973 May 18;243(5403):163–166. doi: 10.1038/243163a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Intracellular calcium and desensitization of acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):447–452. doi: 10.1098/rspb.1980.0106. [DOI] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Boyd N. D., Cohen J. B. Conformations of Torpedo acetylcholine receptor associated with ion transport and desensitization. Biochemistry. 1982 Jul 6;21(14):3460–3467. doi: 10.1021/bi00257a032. [DOI] [PubMed] [Google Scholar]
- Noble M. D., Brown T. H., Peacock J. H. Regulation of acetylcholine receptor levels by a cholinergic agonist in mouse muscle cell cultures. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3488–3492. doi: 10.1073/pnas.75.7.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., Stallcup B. alpha-Bungarotoxin binding and cholinergic receptor function on a rat sympathetic nerve line. J Biol Chem. 1977 Dec 10;252(23):8629–8633. [PubMed] [Google Scholar]
- Patrick J., Stallcup W. B. Immunological distinction between acetylcholine receptor and the alpha-bungarotoxin-binding component on sympathetic neurons. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4689–4692. doi: 10.1073/pnas.74.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Bradley R. J., Dreyer F. The acetylcholine receptor at the neuromuscular junction. Physiol Rev. 1982 Oct;62(4 Pt 1):1271–1340. doi: 10.1152/physrev.1982.62.4.1271. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Role L. W. Substance P modulation of acetylcholine-induced currents in embryonic chicken sympathetic and ciliary ganglion neurons. Proc Natl Acad Sci U S A. 1984 May;81(9):2924–2928. doi: 10.1073/pnas.81.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization and recovery at the frog neuromuscular junction. J Gen Physiol. 1977 Apr;69(4):431–447. doi: 10.1085/jgp.69.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S., Taylor P. Functional consequences of agonist-mediated state transitions in the cholinergic receptor. Studies in cultured muscle cells. J Biol Chem. 1979 May 10;254(9):3315–3325. [PubMed] [Google Scholar]
- Stallcup W. B., Patrick J. Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):634–638. doi: 10.1073/pnas.77.1.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B. Sodium and calcium fluxes in a clonal nerve cell line. J Physiol. 1979 Jan;286:525–540. doi: 10.1113/jphysiol.1979.sp012635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G., Georgia B., Lappi S., Chignell C. F., Taylor P. Kinetics of agonist-mediated transitions in state of the cholinergic receptor. J Biol Chem. 1977 Nov 10;252(21):7648–7656. [PubMed] [Google Scholar]


