Abstract
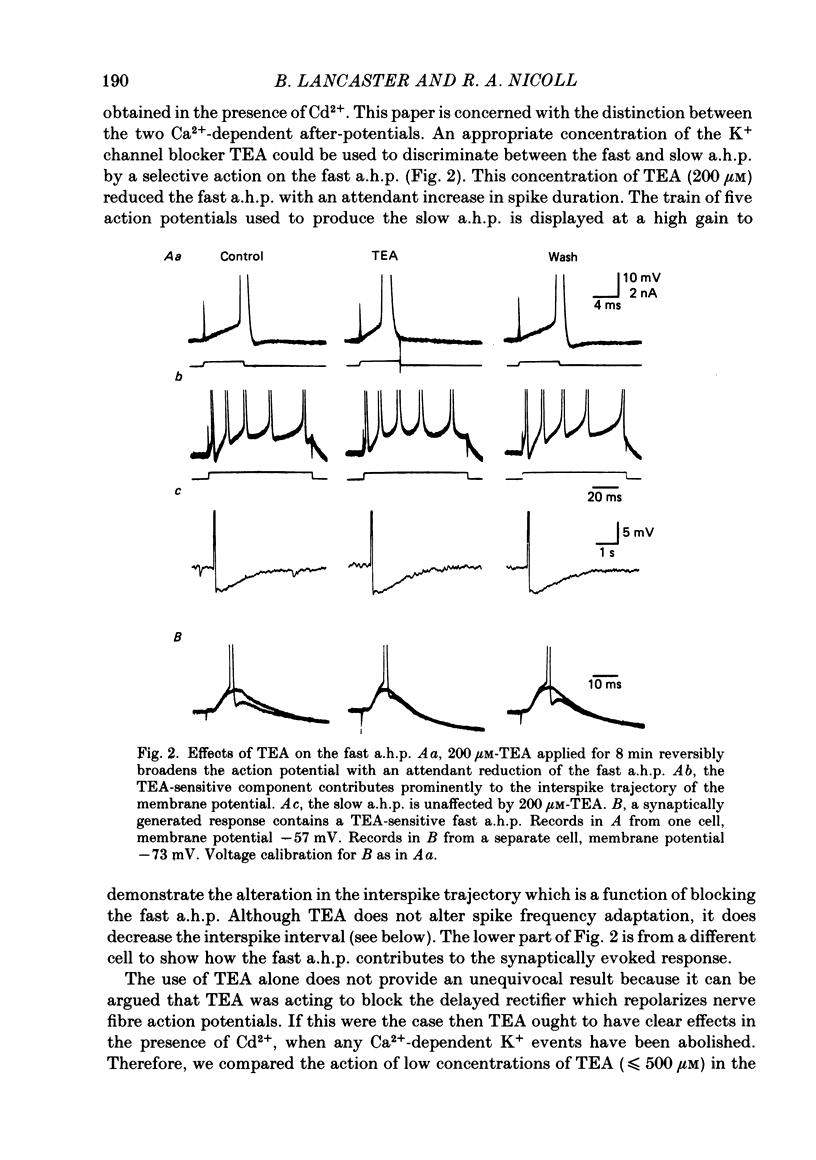
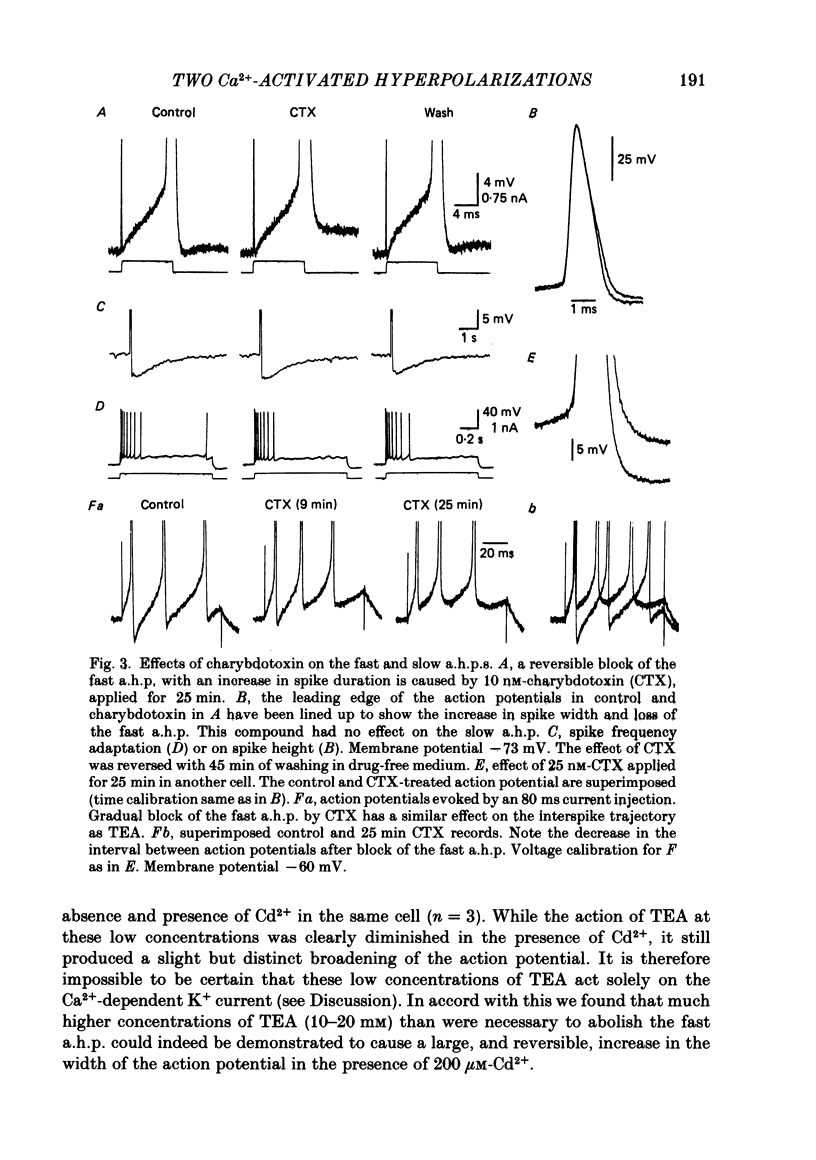
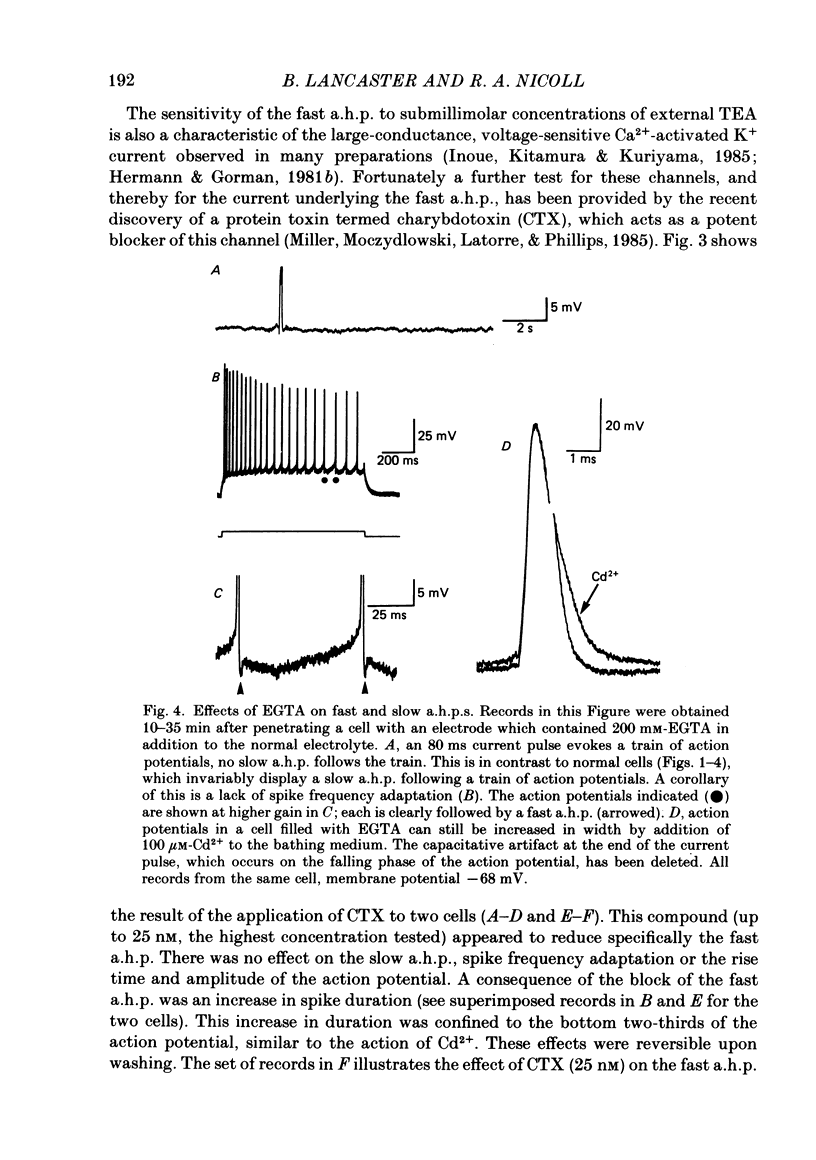
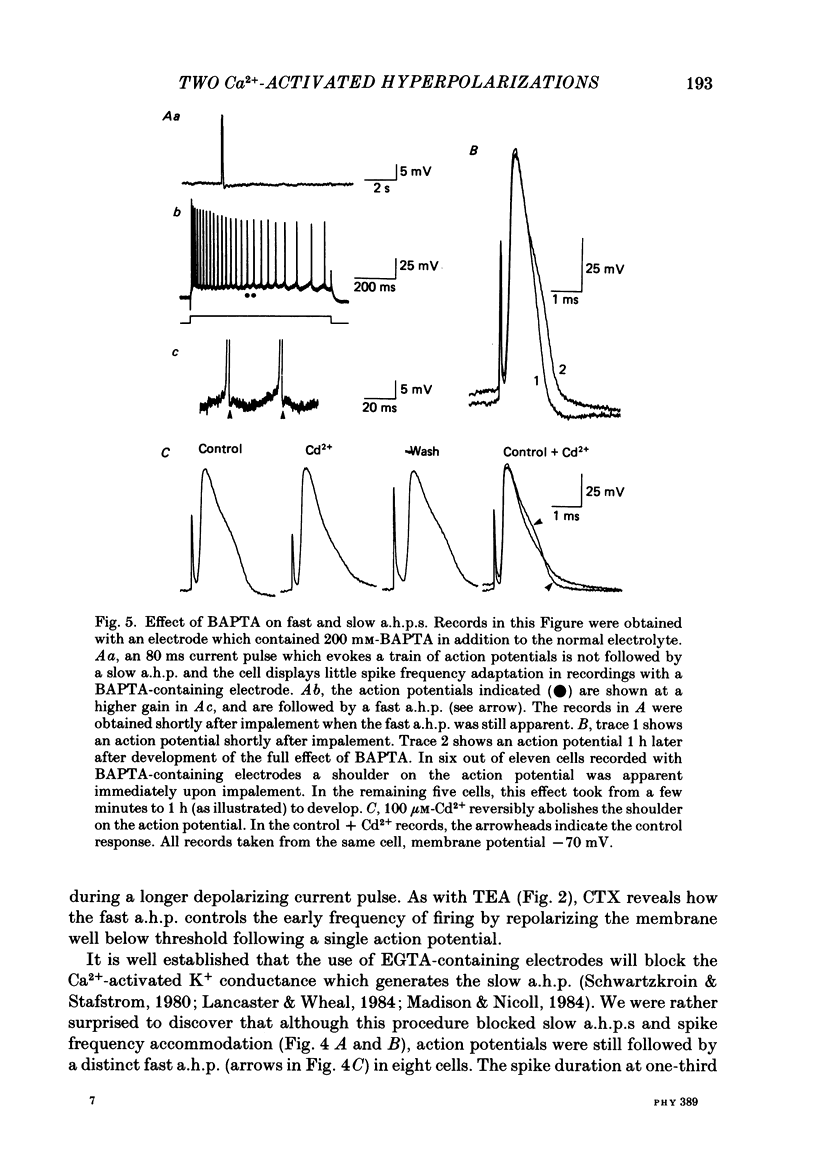
1. Intracellular recording from hippocampal CA1 pyramidal cells in the slice preparation was used to analyse the pharmacological sensitivity of action potential repolarization and the hyperpolarizations that follow the action potential. The Ca2+-activated after-hyperpolarizations (a.h.p.s) could be divided into a fast a.h.p. with a time course of milliseconds, and a slow a.h.p. which lasted for a few seconds at a temperature of 30 degrees C. 2. The repolarization of the action potential is sensitive to the Ca2+ channel blocker Cd2+. This effect is simultaneous with a block of the fast a.h.p. which follows immediately upon the repolarization of the action potential. The slow a.h.p. was also blocked by Cd2+. 3. Low concentrations of the K+ channel blocker, tetraethylammonium (TEA; 200-500 microM), block the fast a.h.p. and slow down action potential repolarization. The slow a.h.p. was not affected by low concentrations of TEA. 4. The action potential repolarization and the fast a.h.p. are also reversibly sensitive to charybdotoxin. This agent had no effect on the slow a.h.p. 5. When EGTA or BAPTA were added to the normal recording electrolyte (KMeSO4), the generation of slow a.h.p.s was prevented. In addition, cells impaled with BAPTA-containing electrodes displayed broader action potentials and much reduced fast a.h.p.s compared to recordings made with electrodes containing KMeSO4 alone or with EGTA. 6. The slow a.h.p. can be eliminated by noradrenaline, 8-bromocyclic AMP or carbachol. Under these conditions there are no effects on the fast a.h.p. or on action potential duration. 7. Block of the fast a.h.p. with TEA or CTX (charybdotoxin) is associated with an increased frequency of the first few action potentials during a depolarization. This is a quite distinct effect from the greatly increased number of action potentials which results from block of the slow a.h.p. 8. The results support a conclusion that the fast a.h.p. is generated by the TEA- and voltage-sensitive Ca2+-activated K+ current, IC. This current is involved in spike repolarization and turns off upon the return to resting potential. Thus block of IC has no effect on the slow a.h.p. which is caused by a separate membrane current.
Full text
PDF


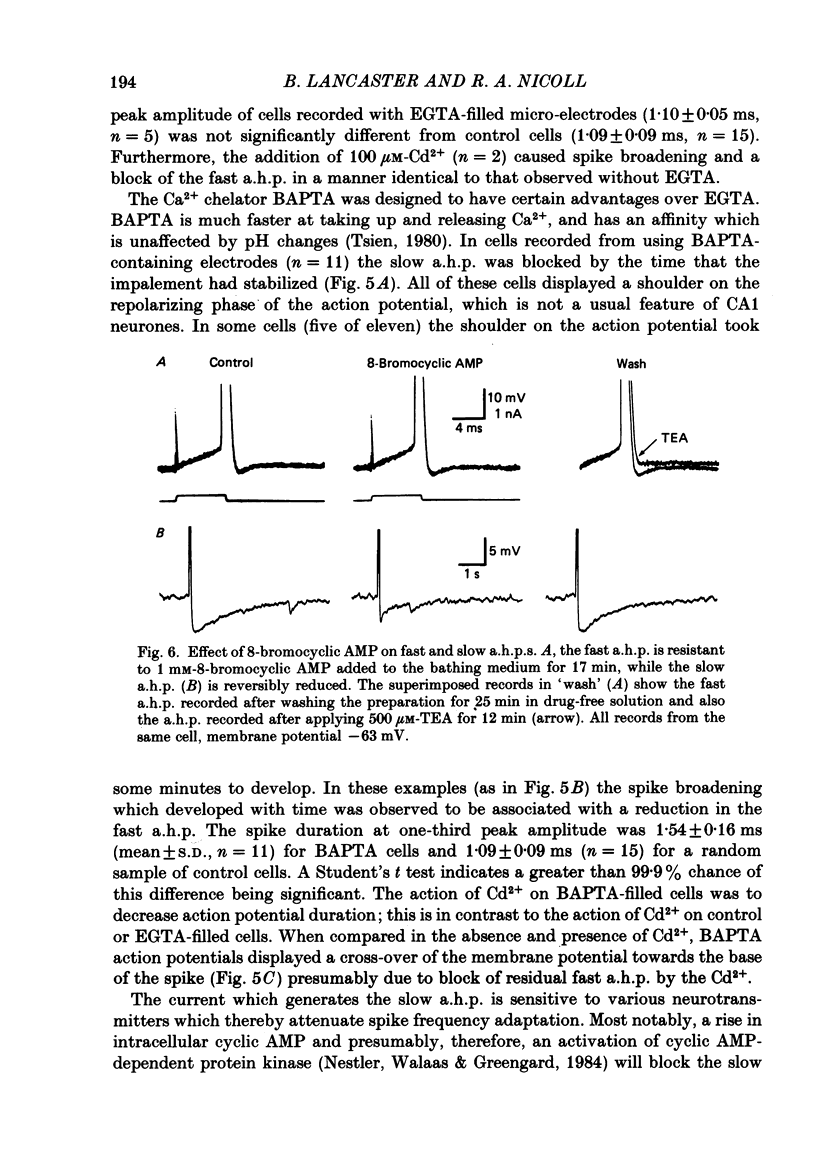

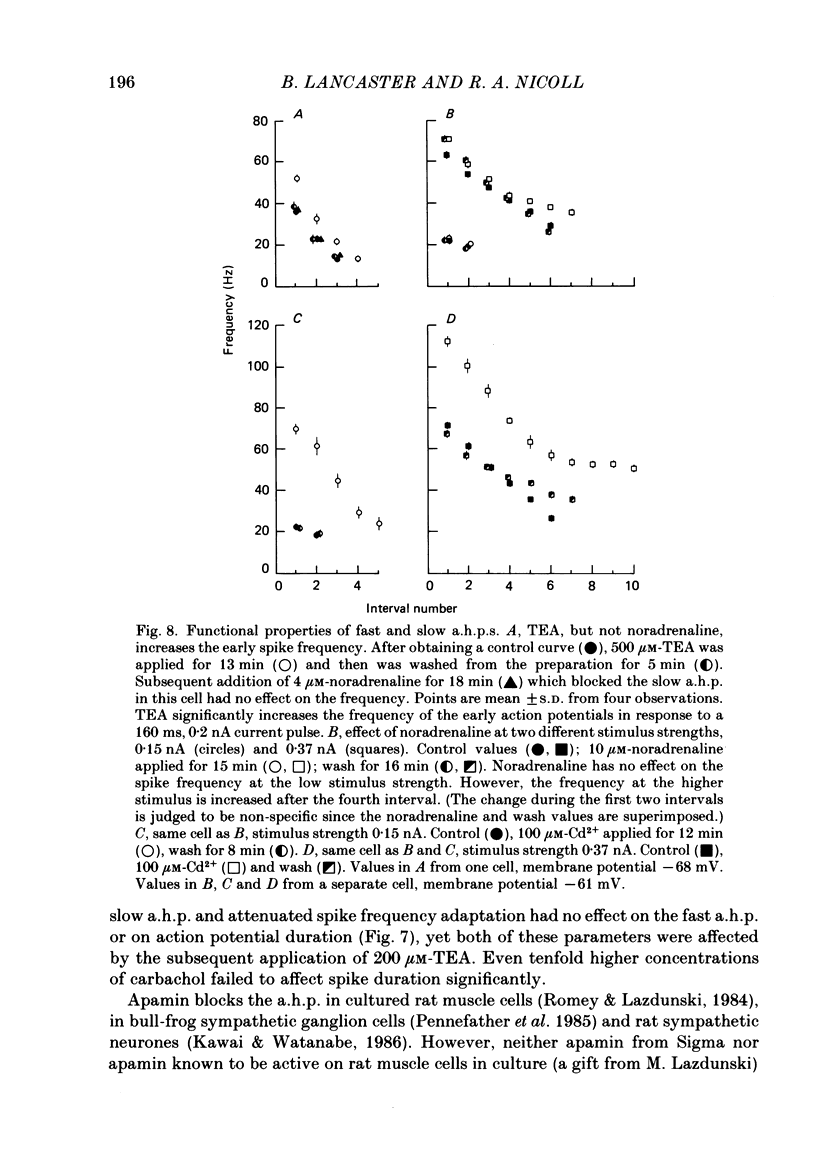







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984 Jul;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Gage P. W., Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol. 1984 Nov;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Eckert R. Two components of Ca-dependent potassium current in identified neurons of Aplysia californica. Pflugers Arch. 1985 Apr;403(4):353–359. doi: 10.1007/BF00589246. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Fischbach G. D. The action potential of chick dorsal root ganglion neurones maintained in cell culture. J Physiol. 1977 May;267(2):281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Delayed currents in myelinated nerve fibres of Xenopus laevis investigated with voltage clamp technique. J Physiol. 1962 Jan;160:40–45. doi: 10.1113/jphysiol.1962.sp006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Evidence for two types of afterhyperpolarization in CA1 pyramidal cells in the hippocampus. Brain Res. 1981 Feb 16;206(2):462–468. doi: 10.1016/0006-8993(81)90548-5. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of tetraethylammonium on potassium currents in a molluscan neurons. J Gen Physiol. 1981 Jul;78(1):87–110. doi: 10.1085/jgp.78.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Action of tetraethylammonium on calcium-activated potassium channels in pig pancreatic acinar cells studied by patch-clamp single-channel and whole-cell current recording. J Membr Biol. 1985;86(2):139–144. doi: 10.1007/BF01870780. [DOI] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Wheal H. V. The synaptically evoked late hyperpolarisation in hippocampal CA1 pyramidal cells is resistant to intracellular EGTA. Neuroscience. 1984 May;12(1):267–275. doi: 10.1016/0306-4522(84)90152-0. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Leonard R. J., Yamaguchi K. Acetylcholine raises excitability by inhibiting the fast transient potassium current in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1986 May;83(9):3022–3026. doi: 10.1073/pnas.83.9.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Walaas S. I., Greengard P. Neuronal phosphoproteins: physiological and clinical implications. Science. 1984 Sep 21;225(4668):1357–1364. doi: 10.1126/science.6474180. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Lazdunski M. The coexistence in rat muscle cells of two distinct classes of Ca2+-dependent K+ channels with different pharmacological properties and different physiological functions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):669–674. doi: 10.1016/0006-291x(84)91355-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Thomas M. V. Voltage-clamp analysis of a calcium-mediated potassium conductance in cockroach (Periplaneta americana) central neurones. J Physiol. 1984 May;350:159–178. doi: 10.1113/jphysiol.1984.sp015194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Vergara C., Moczydlowski E., Latorre R. Conduction, Blockade and Gating in a Ca -activated K Channel Incorporated into Planar Lipid Bilayers. Biophys J. 1984 Jan;45(1):73–76. doi: 10.1016/S0006-3495(84)84114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. S., Lecar H., Adler M. Single calcium-dependent potassium channels in clonal anterior pituitary cells. Biophys J. 1982 Sep;39(3):313–317. doi: 10.1016/S0006-3495(82)84522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbicz K. L., Weight F. F. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985 Apr;53(4):1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]


