Abstract
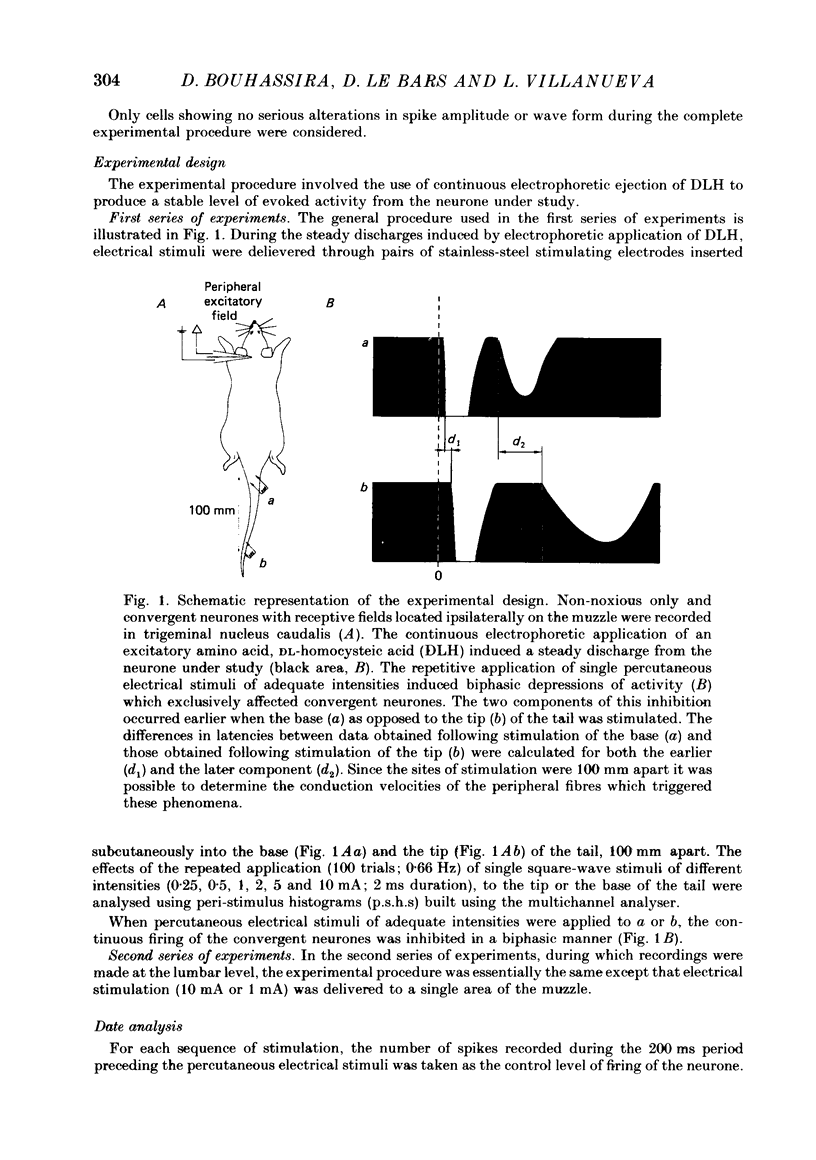
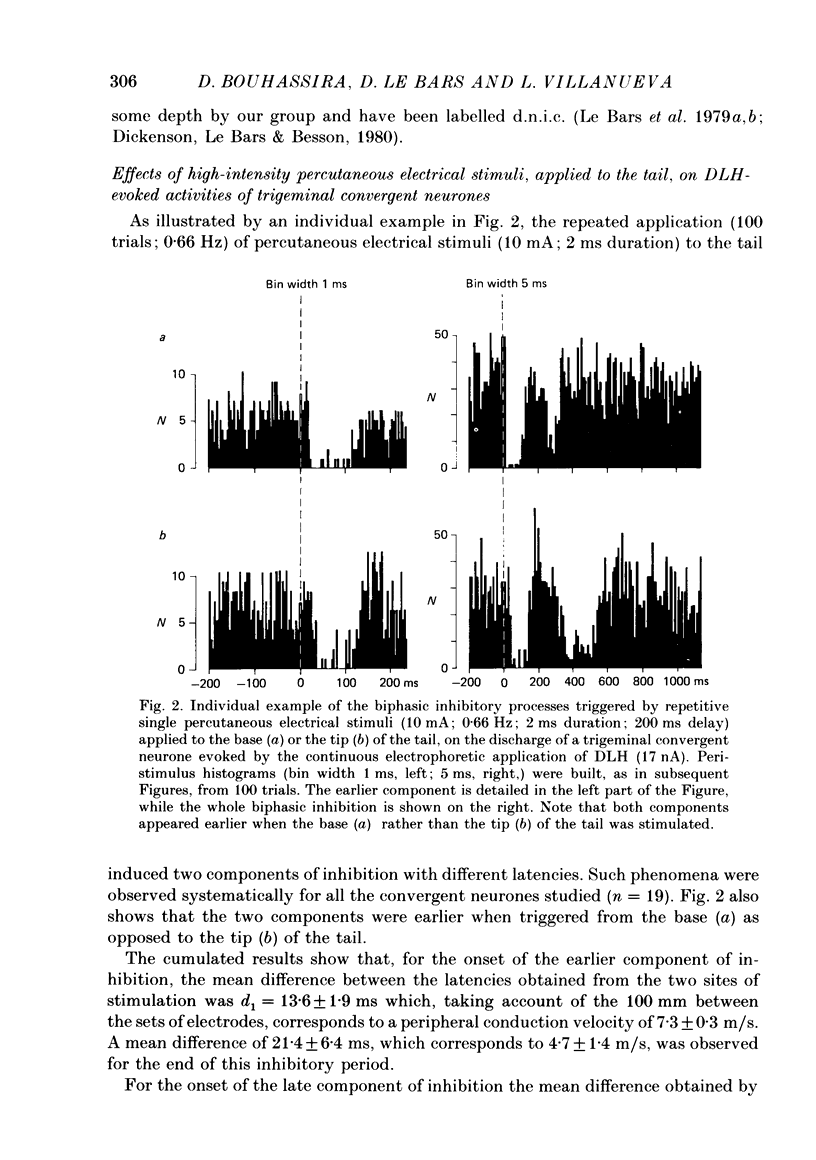
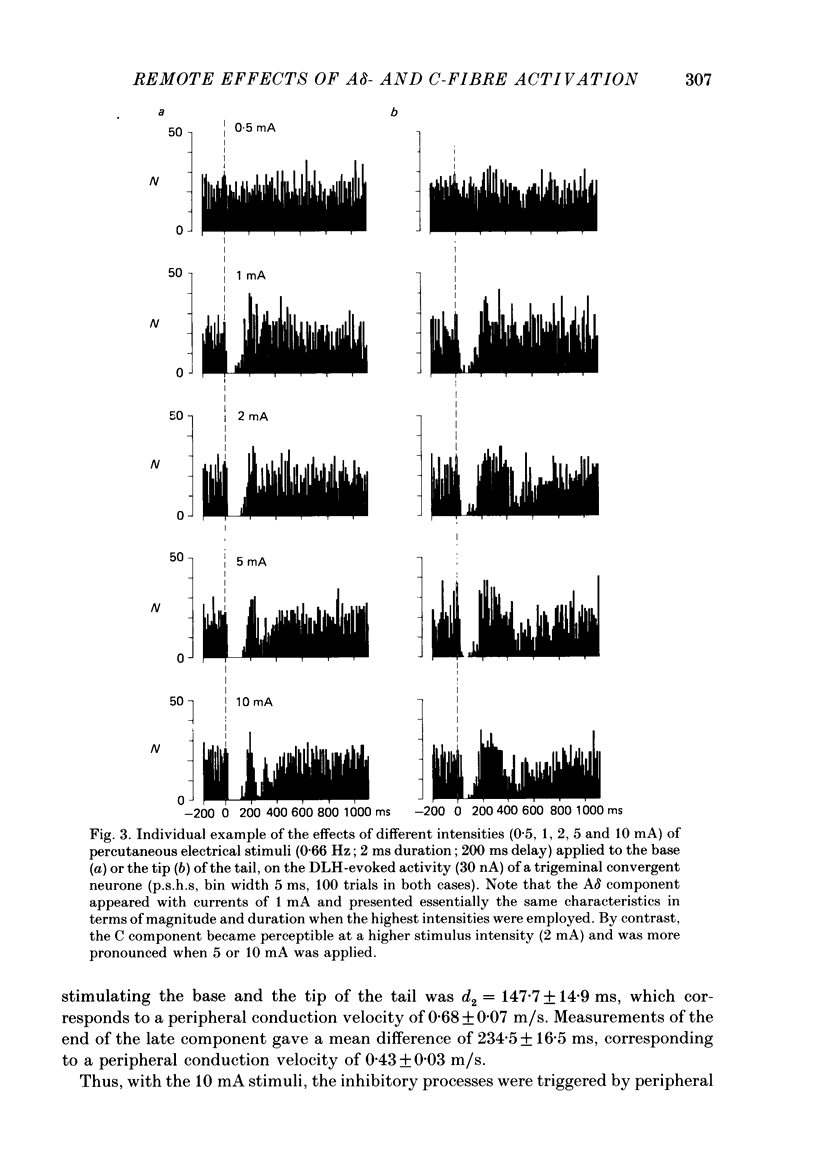
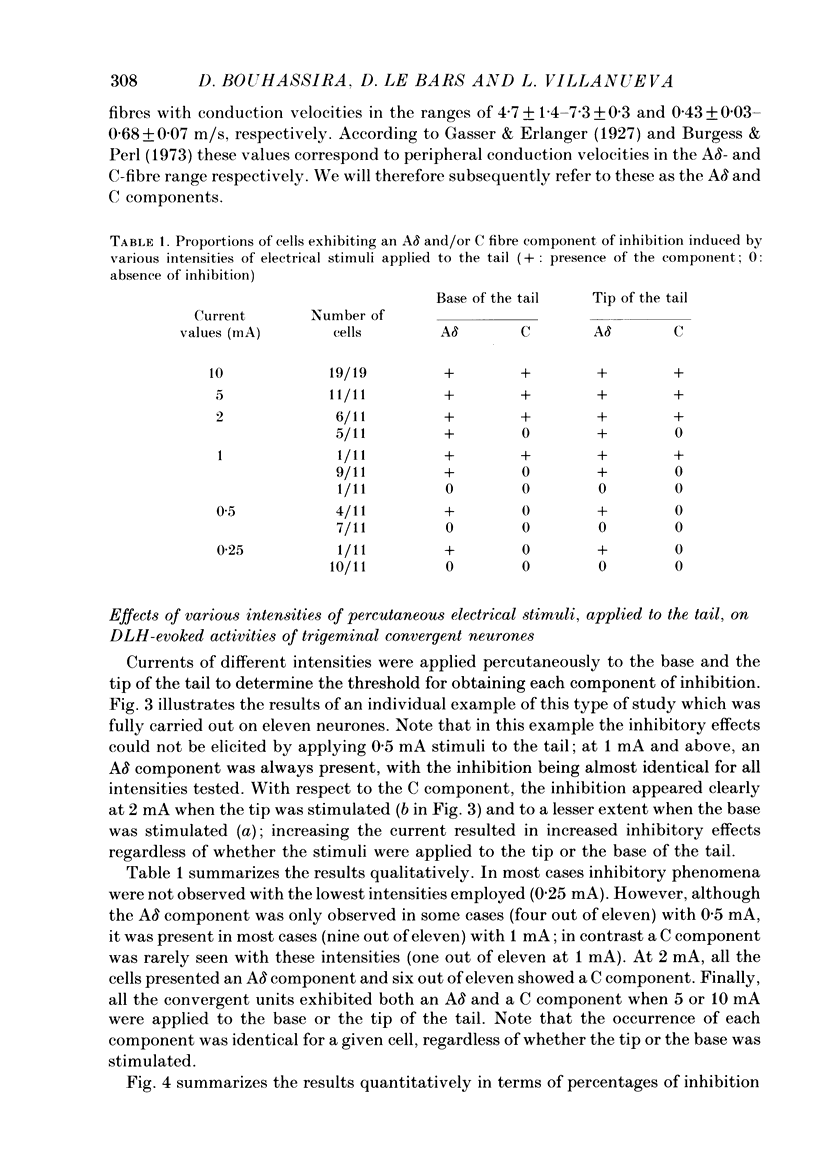
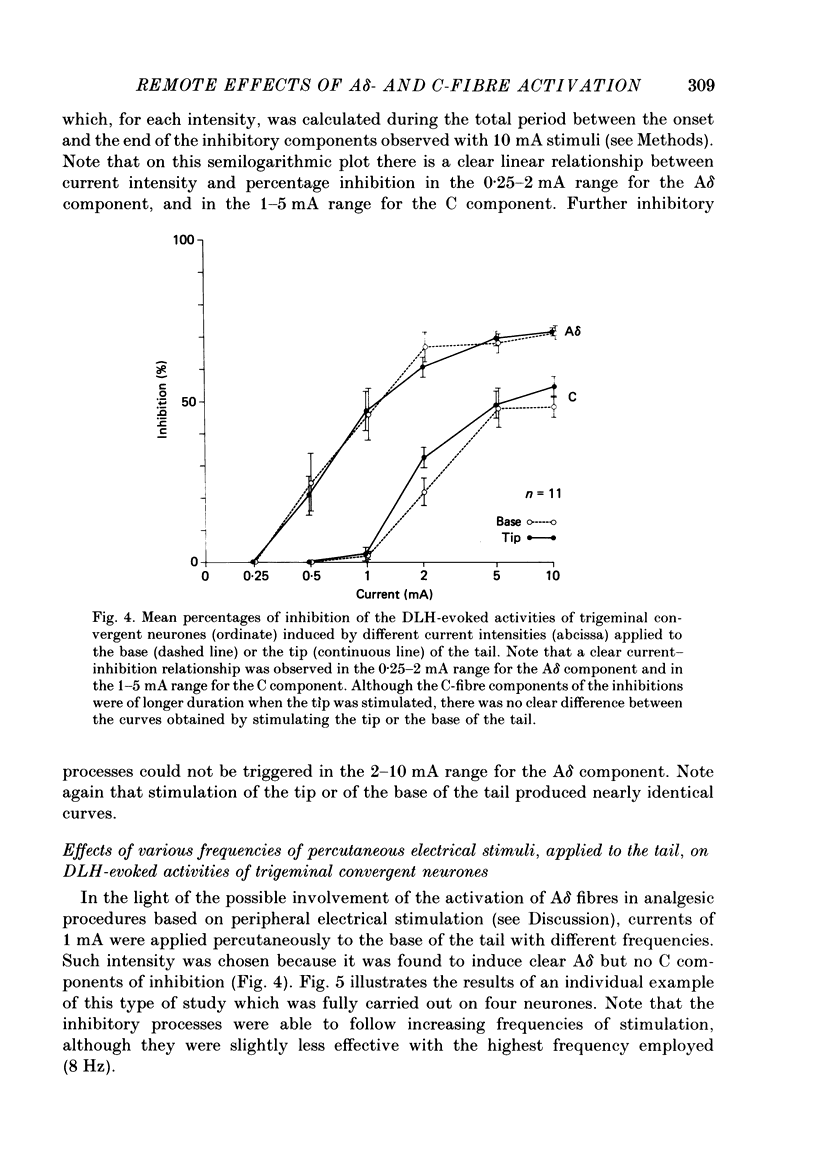
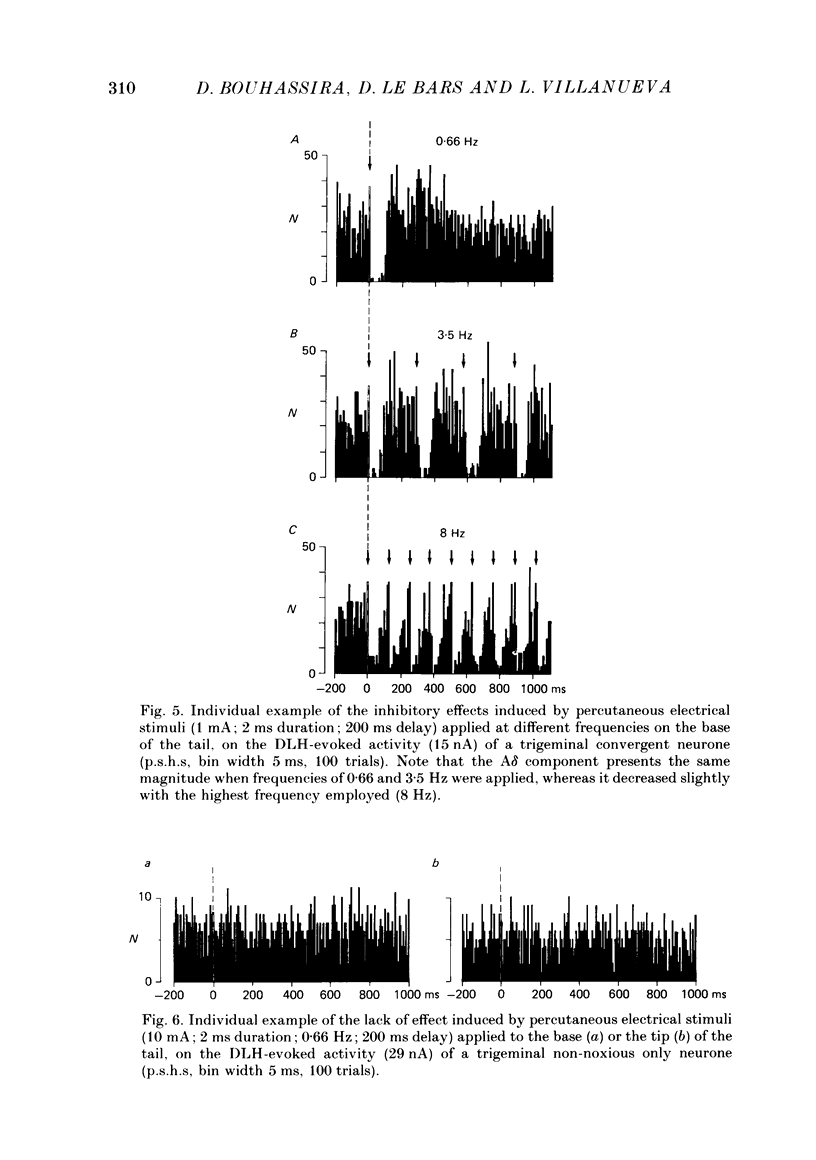
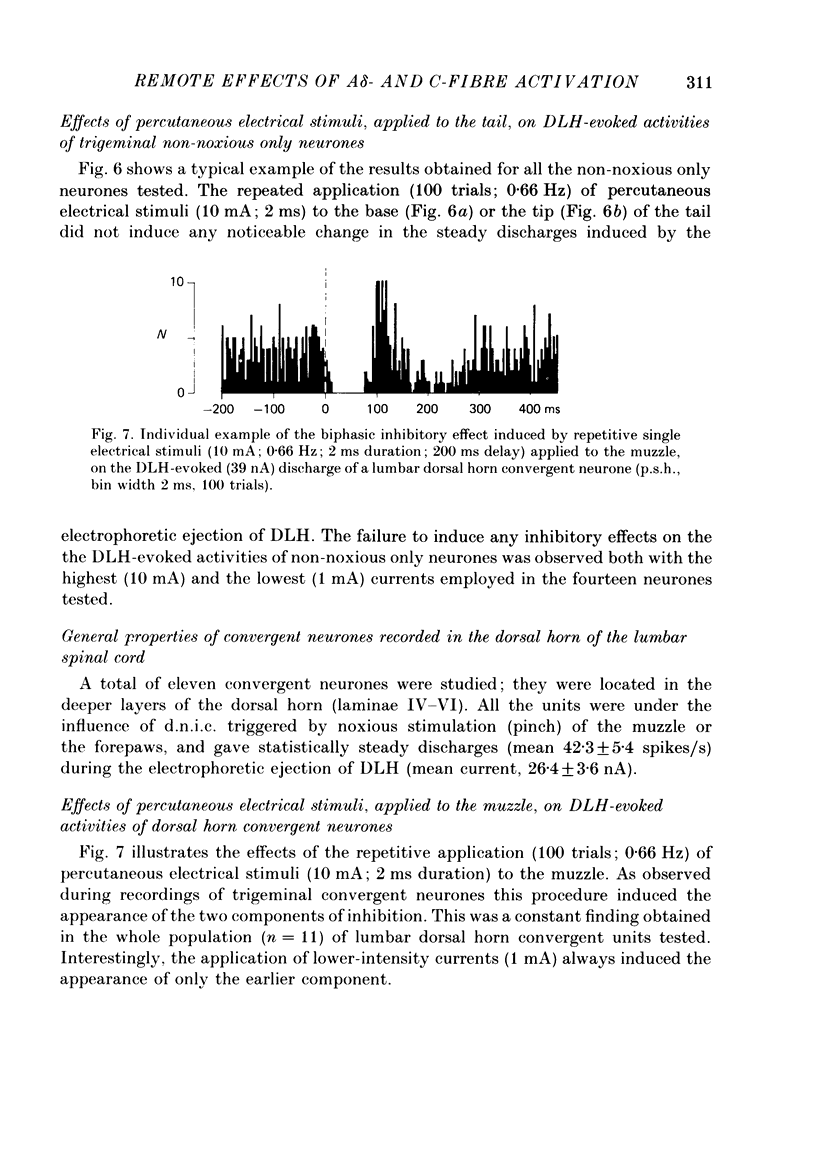
1. Extracellular recordings were made from fourteen non-noxious only and nineteen convergent neurones in trigeminal nucleus caudalis of halothane-anaesthetized rats. All the neurones studied were excited by the continuous micro-electrophoretic ejection of an excitatory amino acid, DL-homocysteic acid (DLH), with mean currents of 38.0 +/- 7.2 and 39.8 + 6.5 nA producing steady discharges of 35.0 +/- 3.3 and 31.8 +/- 1.3 spikes/s from the non-noxious only and convergent neurones respectively. 2. The repeated percutaneous application (100 trials; 0.66 Hz) of single square-wave stimuli (10 mA; 2 ms) to the tail always induced a biphasic depression of the activity of the convergent, but never of the non-noxious only, neurones. Both the early and late components of this inhibition occurred at shorter latencies when the base rather than the tip of the tail was stimulated. Differences in latencies from the two sites of stimulation (100 mm apart) were used to estimate the conduction velocities of the peripheral fibres which were triggering the inhibitions. 3. The cumulated results showed that, for the onset of the earlier component of the inhibition, the mean difference between the latencies from the two sites of stimulation was 13.6 +/- 1.9 ms, corresponding to a peripheral conduction velocity of 7.3 +/- 0.3 m/s, which is in the A delta-fibre range. For the onset of the late component of inhibition, the mean difference was 147.7 +/- 14.9 ms, corresponding to a peripheral conduction velocity of 0.68 +/- 0.07 m/s, which is in the C-fibre range. 4. When currents of different intensities were applied percutaneously to the two stimulation sites, the thresholds for obtaining the A delta component were in the range 0.25-0.5 mA whereas the C component appeared with currents 1-2 mA. A clear relationship between current intensity and magnitude of inhibition was observed in the 0.25-2 mA range for the A delta component and in the 1-5 mA range for the C component. 5. In an additional series of experiments recordings were made from eleven convergent neurones in the dorsal horn of the lumbar spinal cord. By using essentially the same experimental procedure the effects of repetitive application (100 trials, 0.66 Hz) of percutaneous electrical stimuli (1 or 10 mA, 2 ms) applied to the muzzle, were studied on the steady discharges (42.3 +/- 5.4 spikes/s) induced by DLH. The application of the 10 mA stimuli induced a biphasic depression of activity, whereas only an early component was observed following 1 mA stimuli.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaensen H., Gybels J., Handwerker H. O., Van Hees J. Response properties of thin myelinated (A-delta) fibers in human skin nerves. J Neurophysiol. 1983 Jan;49(1):111–122. doi: 10.1152/jn.1983.49.1.111. [DOI] [PubMed] [Google Scholar]
- Andersson S. A., Holmgren E. On acupuncture analgesia and the mechanism of pain. Am J Chin Med (Gard City N Y) 1975 Oct;3(4):311–334. doi: 10.1142/s0192415x75000396. [DOI] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W., Schaffner R. The differential effects of 5-hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non-nociceptive dorsal horn interneurones in the cat. Brain Res. 1978 Aug 4;151(2):307–321. doi: 10.1016/0006-8993(78)90887-9. [DOI] [PubMed] [Google Scholar]
- Cadden S. W., Villanueva L., Chitour D., Le Bars D. Depression of activities of dorsal horn convergent neurones by propriospinal mechanisms triggered by noxious inputs; comparison with diffuse noxious inhibitory controls (DNIC). Brain Res. 1983 Sep 19;275(1):1–11. doi: 10.1016/0006-8993(83)90412-2. [DOI] [PubMed] [Google Scholar]
- Chung J. M., Lee K. H., Hori Y., Endo K., Willis W. D. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain. 1984 Jul;19(3):277–293. doi: 10.1016/0304-3959(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Le Bars D., Besson J. M. Diffuse noxious inhibitory controls (DNIC). Effects on trigeminal nucleus caudalis neurones in the rat. Brain Res. 1980 Nov 3;200(2):293–305. doi: 10.1016/0006-8993(80)90921-x. [DOI] [PubMed] [Google Scholar]
- Dubner R., Bennett G. J. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Freminet A., Bursaux E., Poyart C. F. Mesure de la vitesse de renouvellement du lactate chez le rat par perfusion de 14-C-U (L) lactate. Pflugers Arch. 1972;334(4):293–302. doi: 10.1007/BF00592164. [DOI] [PubMed] [Google Scholar]
- Gybels J., Handwerker H. O., Van Hees J. A comparison between the discharges of human nociceptive nerve fibres and the subject's ratings of his sensations. J Physiol. 1979 Jul;292:193–206. doi: 10.1113/jphysiol.1979.sp012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan L. M., Kenshalo D. R., Jr, Martin R. F., Haber L. H., Willis W. D. Depression of primate spinothalamic tract neurons by iontophoretic application of 5-hydroxytryptamine. Pain. 1978 Aug;5(2):135–142. doi: 10.1016/0304-3959(78)90035-0. [DOI] [PubMed] [Google Scholar]
- Kawakita K., Funakoshi M. Suppression of the jaw-opening reflex by conditioning a-delta fiber stimulation and electroacupuncture in the rat. Exp Neurol. 1982 Nov;78(2):461–465. doi: 10.1016/0014-4886(82)90063-2. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Campbell J. N. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978 Mar;41(2):509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Chitour D., Clot A. M. The encoding of thermal stimuli by diffuse noxious inhibitory controls (DNIC). Brain Res. 1981 Dec 28;230(1-2):394–399. doi: 10.1016/0006-8993(81)90422-4. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Dickenson A. H., Besson J. M. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979 Jun;6(3):283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Dickenson A. H., Besson J. M. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979 Jun;6(3):305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Chung J. M., Willis W. D., Jr Inhibition of primate spinothalamic tract cells by TENS. J Neurosurg. 1985 Feb;62(2):276–287. doi: 10.3171/jns.1985.62.2.0276. [DOI] [PubMed] [Google Scholar]
- Melzack R., Wall P. D. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Menétrey D., Giesler G. J., Jr, Besson J. M. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977 Jan 18;27(1):15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- Nathan P. W., Rudge P. Testing the gate-control theory of pain in man. J Neurol Neurosurg Psychiatry. 1974 Dec;37(12):1366–1372. doi: 10.1136/jnnp.37.12.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puil E. S-Glutamate: its interactions with spinal neurons. Brain Res. 1981 Dec;228(3):229–322. doi: 10.1016/0165-0173(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Satran R., Goldstein M. N. Pain perception: modification of threshold of intolerance and cortical potentials by cutaneous stimulation. Science. 1973 Jun 15;180(4091):1201–1202. doi: 10.1126/science.180.4091.1201. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. Identification of afferent C units in intact human skin nerves. Brain Res. 1974 Mar 8;67(3):387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., LaMotte R. H., Robinson C. J. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgments of pain and evoked responses in nociceptors with C-fibers. J Neurophysiol. 1984 Feb;51(2):325–339. doi: 10.1152/jn.1984.51.2.325. [DOI] [PubMed] [Google Scholar]
- Van Hees J., Gybels J. M. Pain related to single afferent C fibers from human skin. Brain Res. 1972 Dec 24;48:397–400. doi: 10.1016/0006-8993(72)90198-9. [DOI] [PubMed] [Google Scholar]
- Villanueva L., Cadden S. W., Le Bars D. Diffuse noxious inhibitory controls (DNIC): evidence for post-synaptic inhibition of trigeminal nucleus caudalis convergent neurones. Brain Res. 1984 Oct 29;321(1):165–168. doi: 10.1016/0006-8993(84)90695-4. [DOI] [PubMed] [Google Scholar]
- Villanueva L., Cadden S. W., Le Bars D. Evidence that diffuse noxious inhibitory controls (DNIC) are medicated by a final post-synaptic inhibitory mechanism. Brain Res. 1984 Apr 23;298(1):67–74. doi: 10.1016/0006-8993(84)91147-8. [DOI] [PubMed] [Google Scholar]
- Villanueva L., Le Bars D. The encoding of thermal stimuli applied to the tail of the rat by lowering the excitability of trigeminal convergent neurones. Brain Res. 1985 Mar 25;330(2):245–251. doi: 10.1016/0006-8993(85)90683-3. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Sweet W. H. Temporary abolition of pain in man. Science. 1967 Jan 6;155(3758):108–109. doi: 10.1126/science.155.3758.108. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The gate control theory of pain mechanisms. A re-examination and re-statement. Brain. 1978 Mar;101(1):1–18. doi: 10.1093/brain/101.1.1. [DOI] [PubMed] [Google Scholar]
- Weil-Fugazza J., Godefroy F., Le Bars D. Increase in 5-HT synthesis in the dorsal part of the spinal cord, induced by a nociceptive stimulus: blockade by morphine. Brain Res. 1984 Apr 16;297(2):247–264. doi: 10.1016/0006-8993(84)90566-3. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Mitchell D., Barrett G. D. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain. 1980 Apr;8(2):237–252. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]


