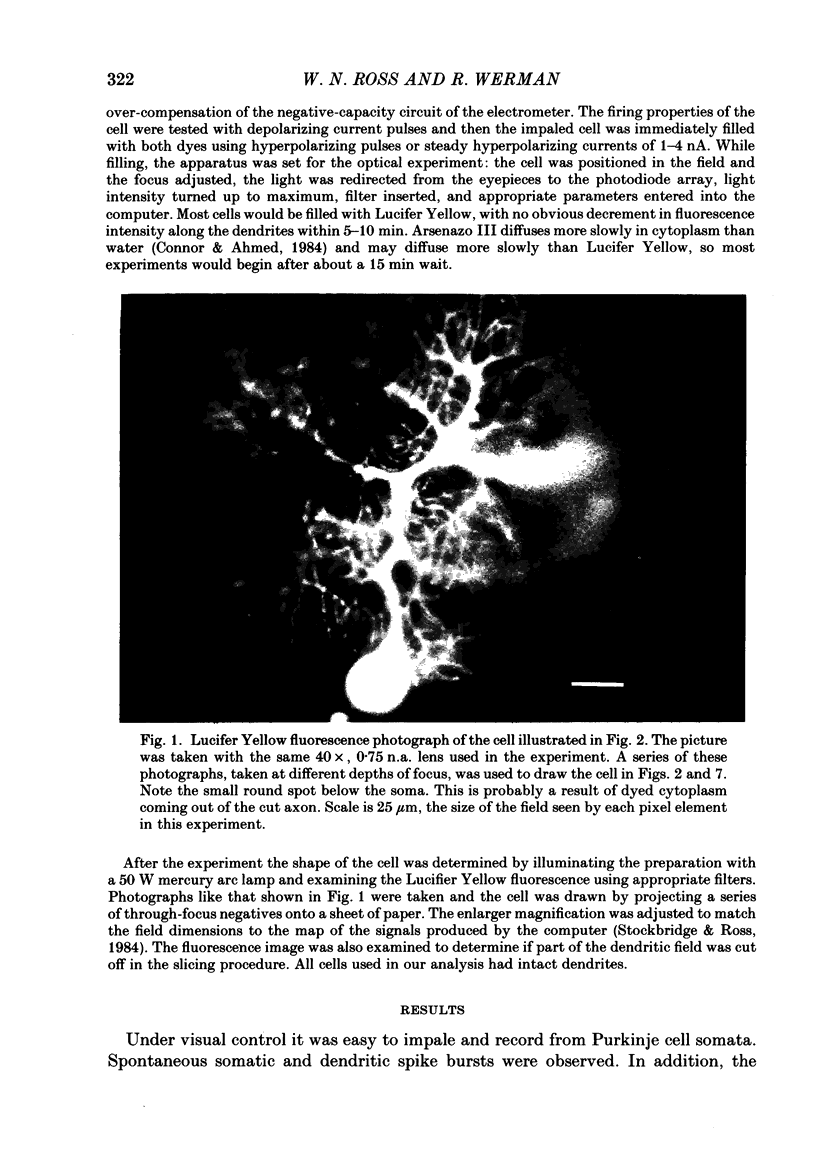
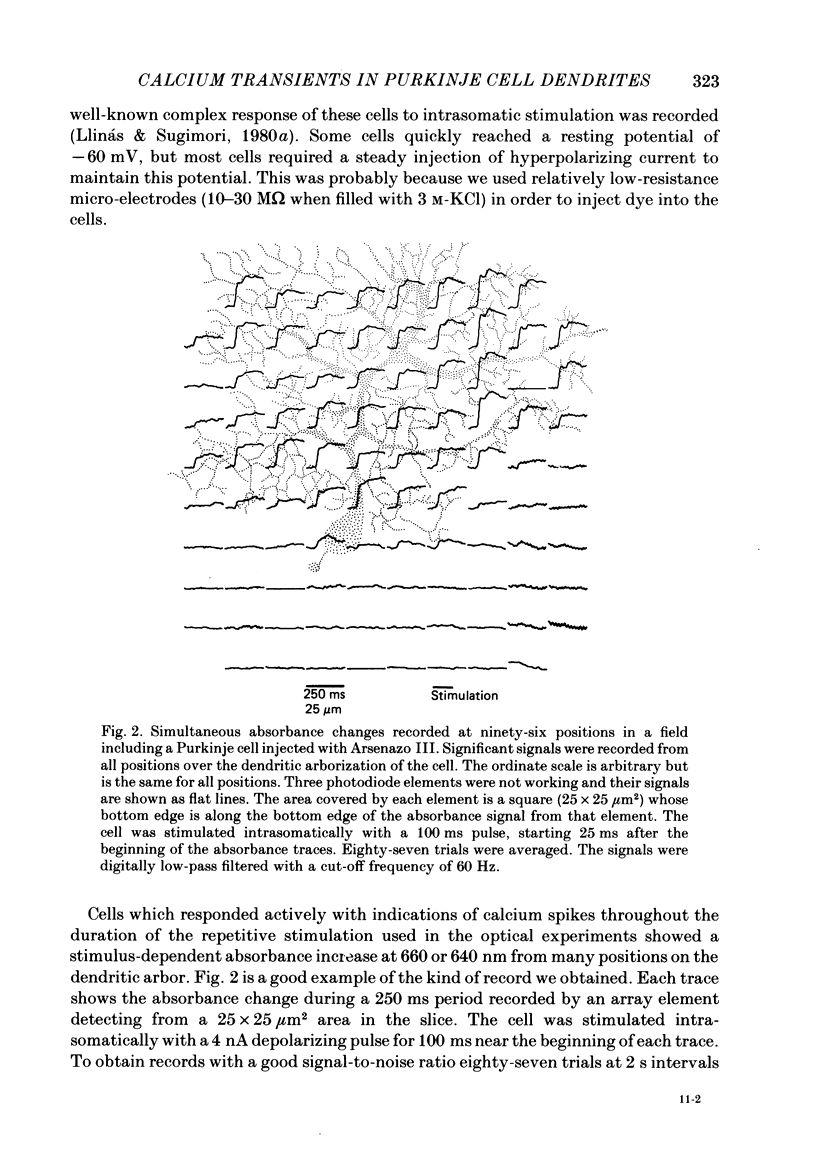
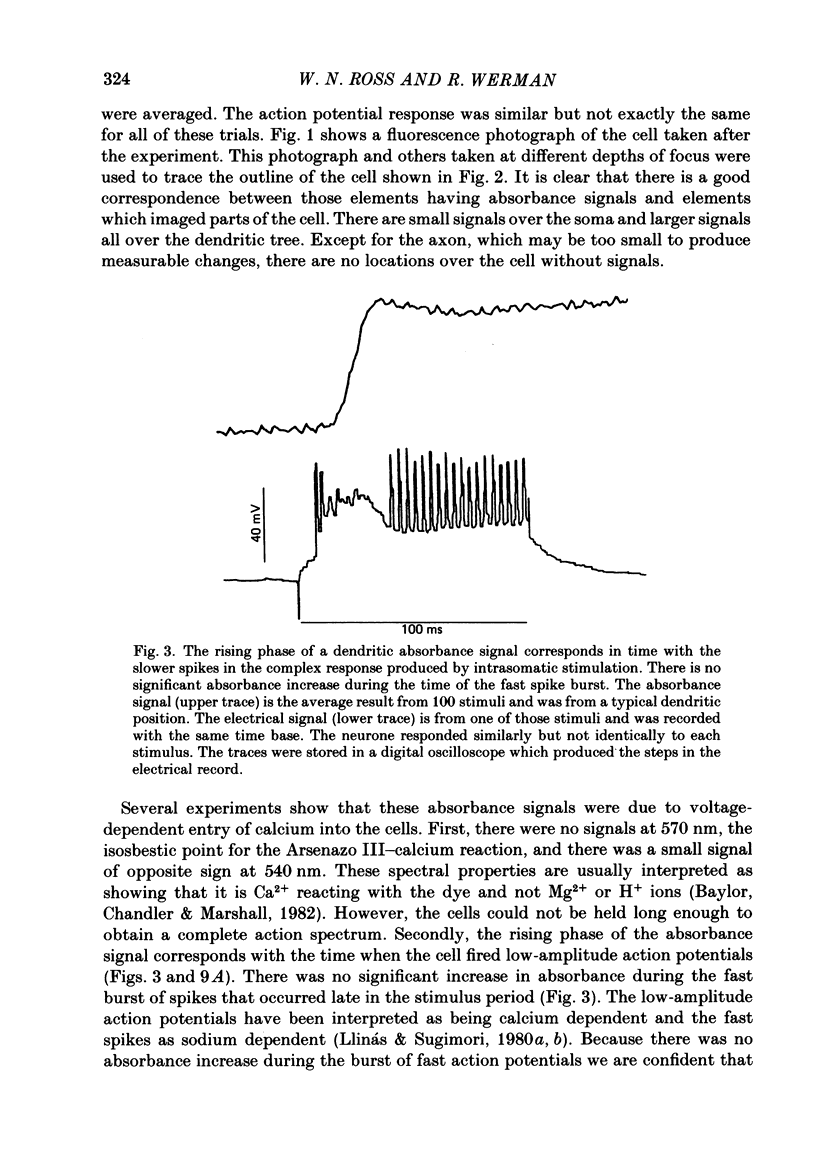
Abstract
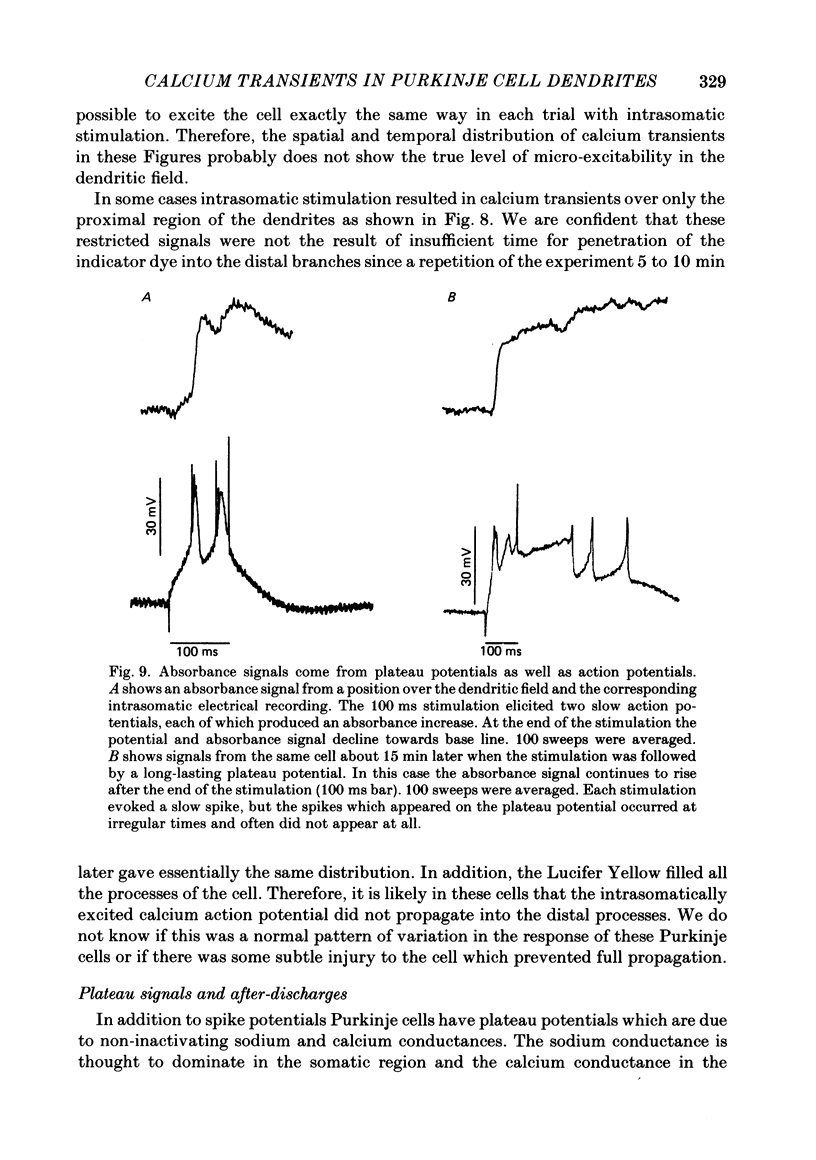
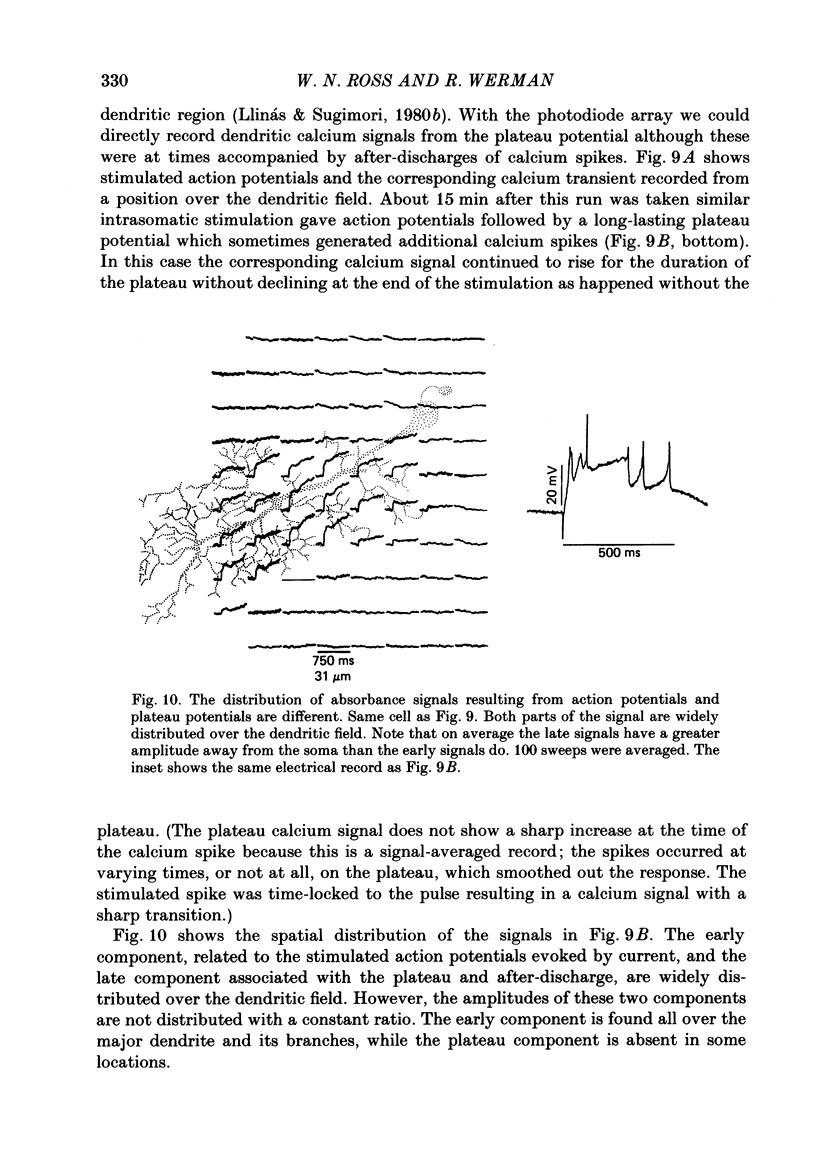
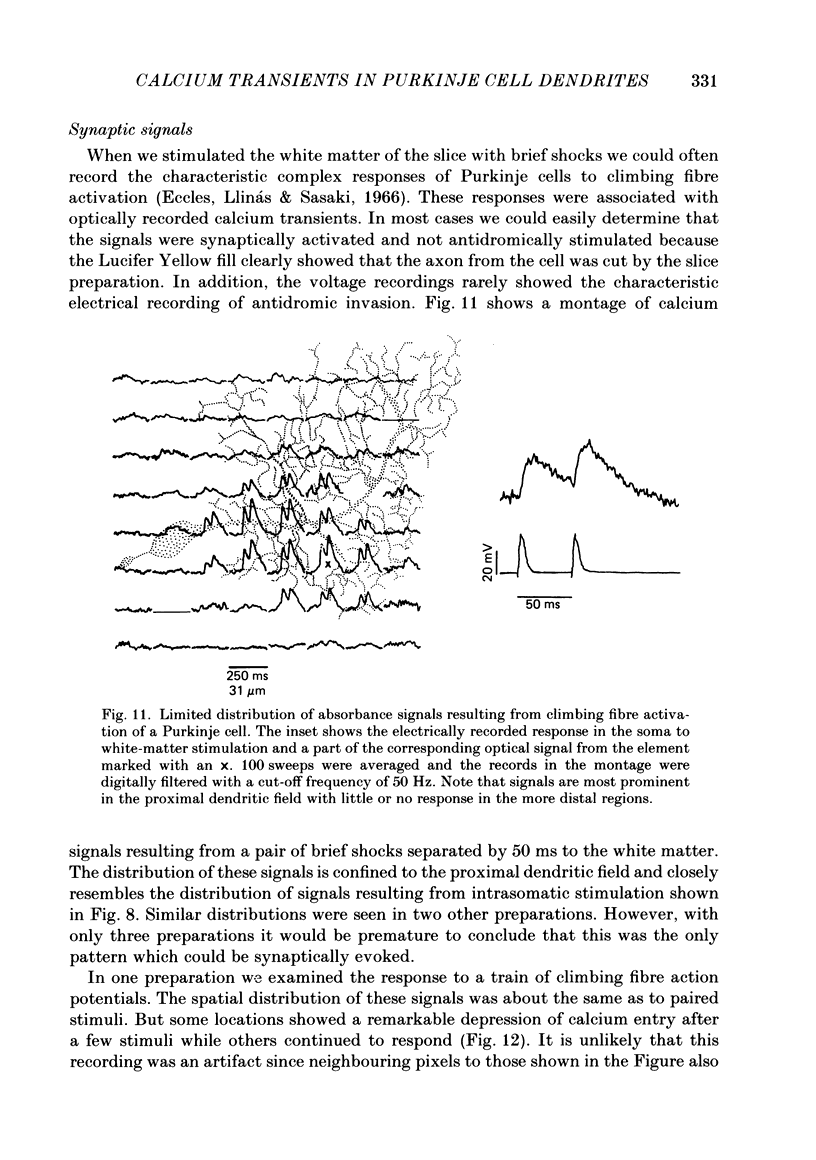
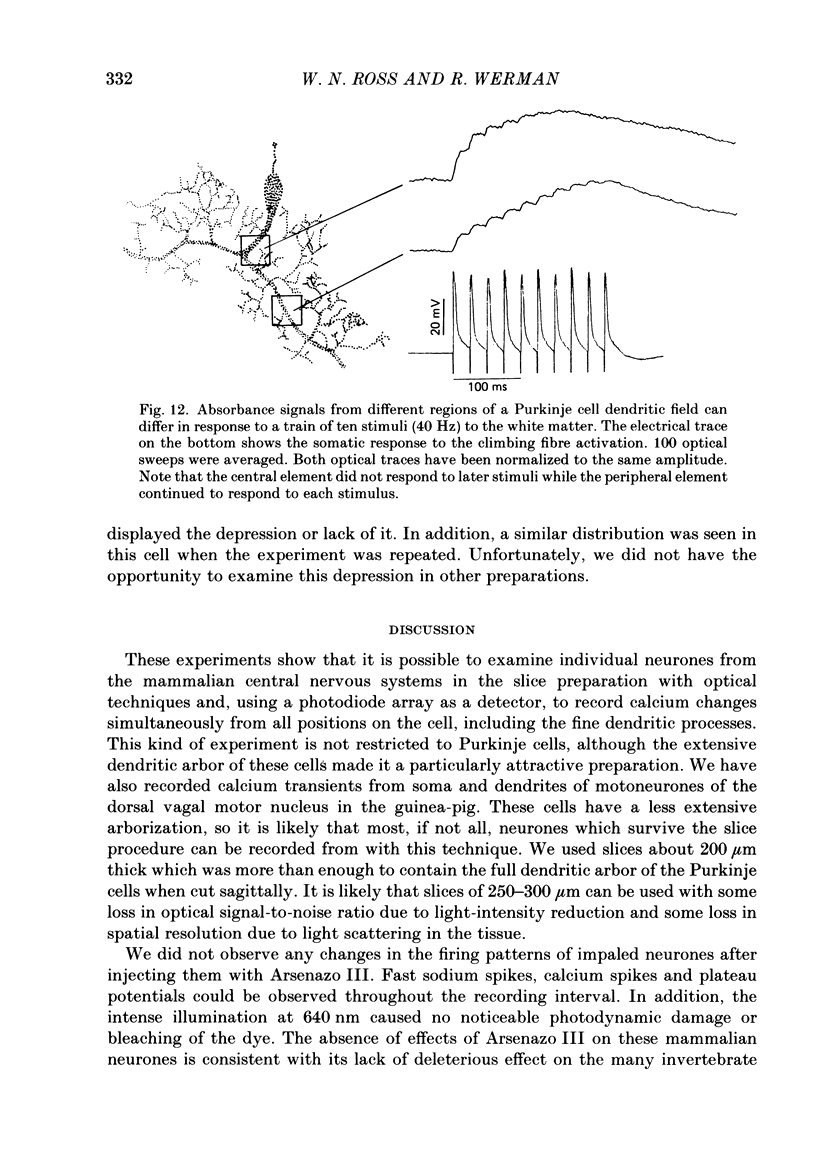
1. A 10 X 10 photodiode array was used to detect stimulation-dependent absorbance changes simultaneously from many positions in the dendrite field of guinea-pig Purkinje cells which had been injected with the calcium indicator Arsenazo III in thin cerebellar slices. Signals from each element of the array were matched to positions on the cells by mapping them onto fluorescence photographs of Lucifer Yellow which had been co-injected into the cells with the Arsenazo III. 2. In response to intrasomatic stimulation the rising phase of the absorbance signals corresponded in time with the calcium spikes recorded with an intracellular electrode. There was no increase in absorbance during bursts of fast sodium spikes. Absorbance signals persisted after the sodium spikes were blocked by tetrodotoxin (TTX). In addition, the signals were largest at 660 nm and small signals of opposite polarity were found at 540 nm. These results indicate that the absorbance signals came from calcium entry into the cell resulting from the turning on of voltage-dependent calcium conductances. 3. In these experiments signals were usually seen all over the dendritic field and were weak or totally absent over the soma. In some cases signals were seen over a more restricted area. With a spatial resolution of 25 microns we were not able to see any evidence for highly localized sites of calcium entry. 4. Sometimes the rising phase of the calcium signals was separated by almost 13 ms in different parts of the dendritic field, too long to be explained by active propagation delay. This suggests that calcium spikes causing these signals can be evoked separately in different regions of the Purkinje cell dendritic field by long-lasting potentials which may reach local threshold at different times. 5. Calcium signals resulting from slow plateau after-potentials and the calcium spikes produced by them were also detected in all locations in the dendritic field. The relative distribution of amplitudes from these plateau signals was different from the distribution of evoked signals during current injection. 6. Climbing fibre synaptic activation produced calcium signals which were distributed over the dendritic arborization, but larger at the main dendritic tree where most of the synaptic contacts are located. 7. Calcium signals were also detected from the dendrites of other neurone types in the in vitro slice preparation. Thus, it is likely that these kind of measurements can be used to analyse the electroresponsiveness of many kinds of neurones in the mammalian brain.
Full text
PDF


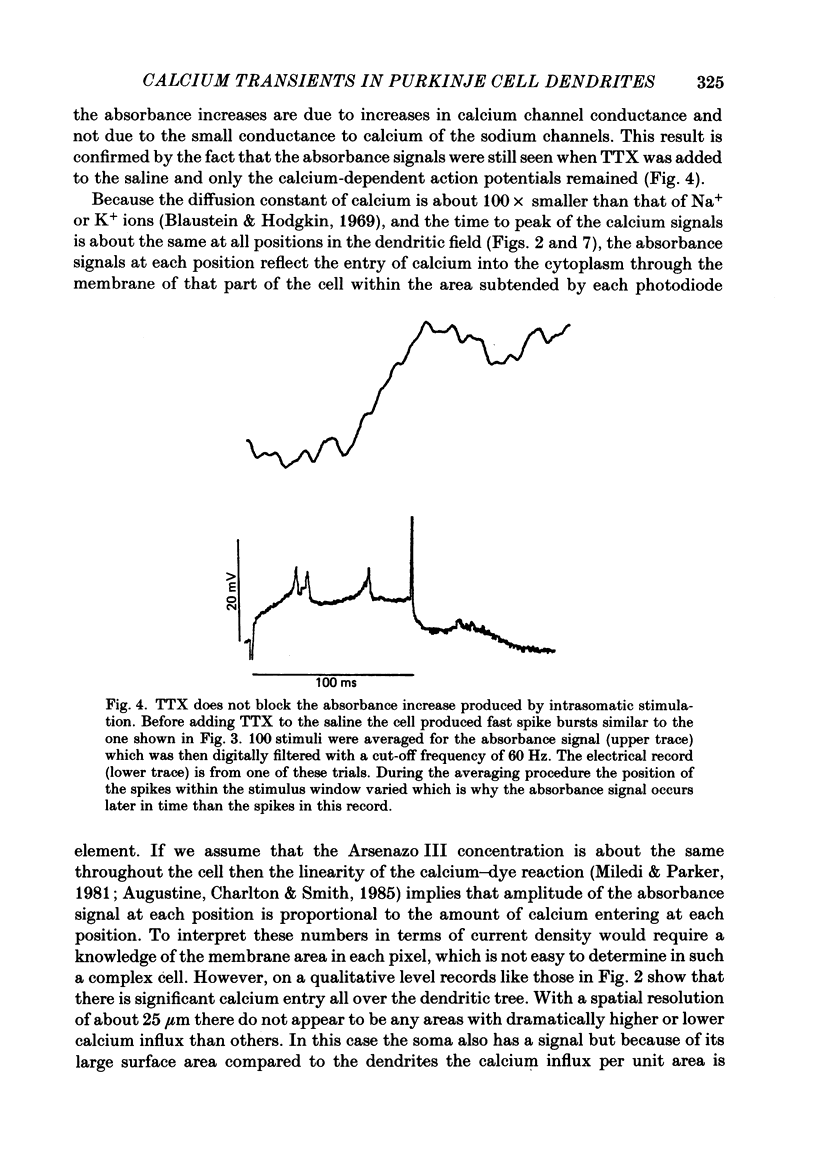
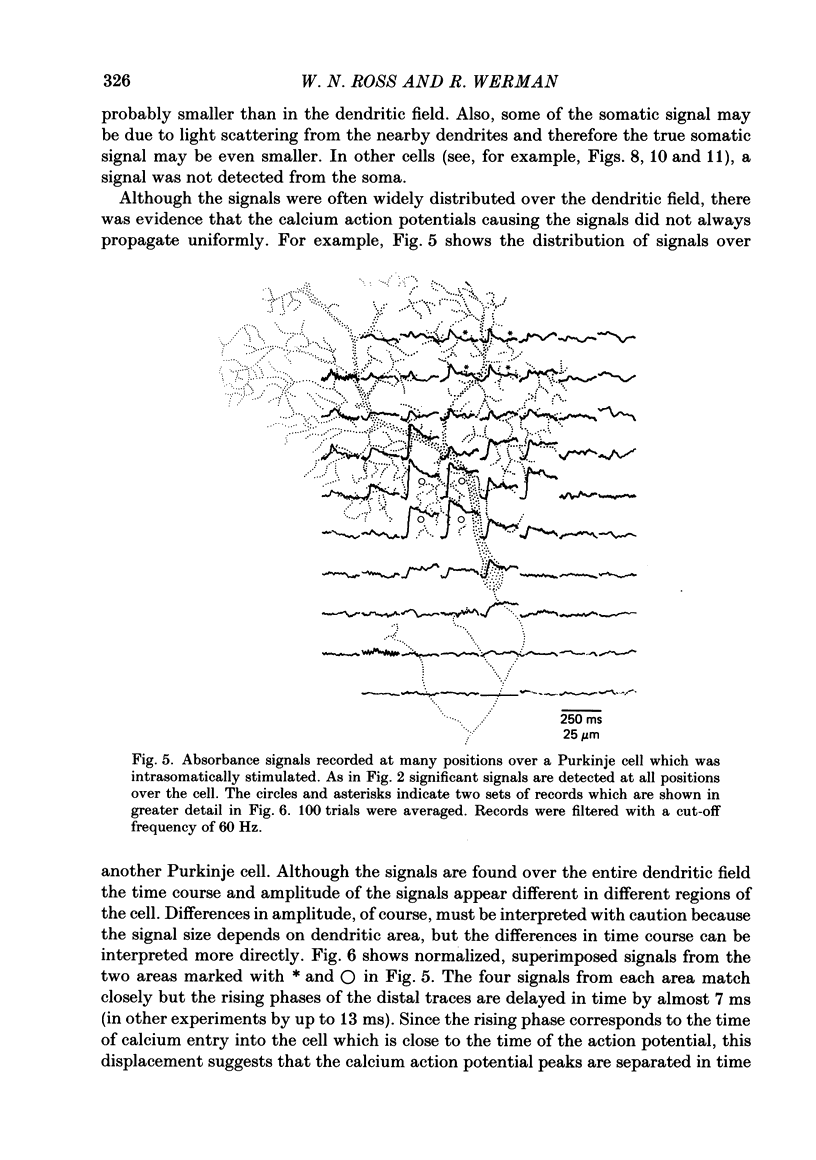
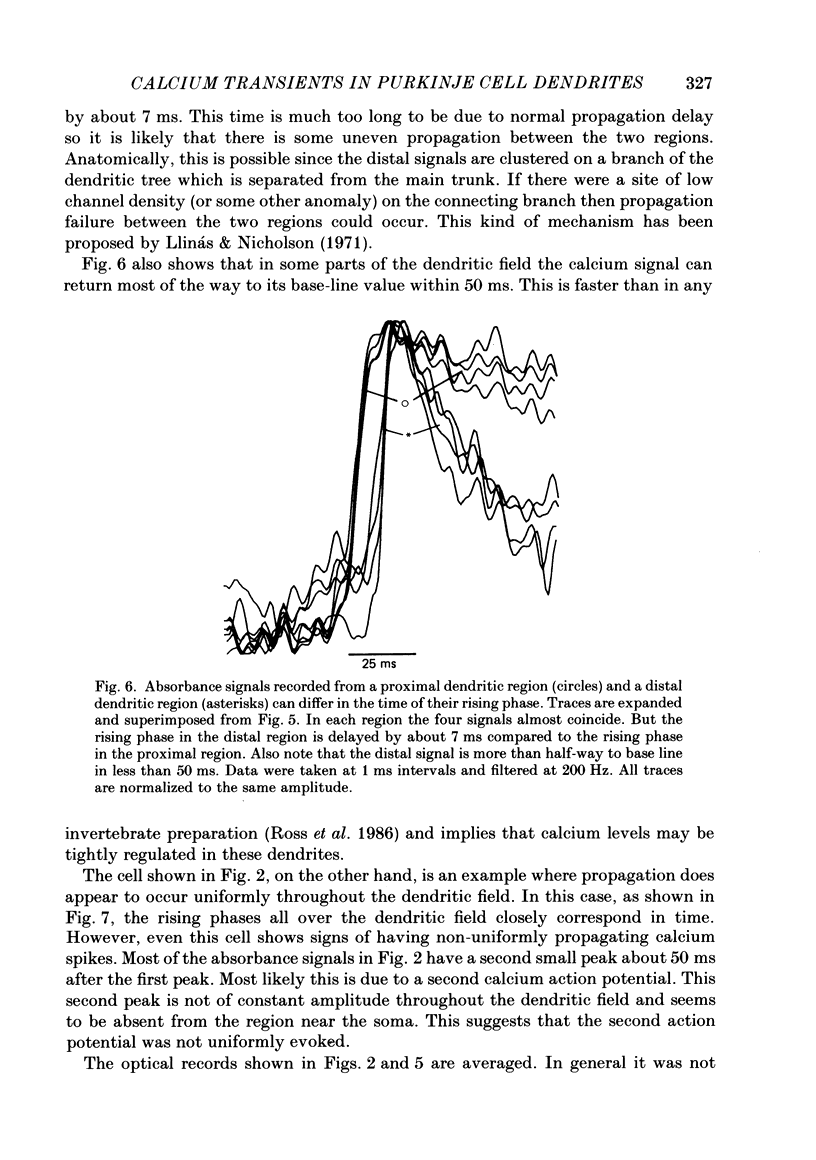
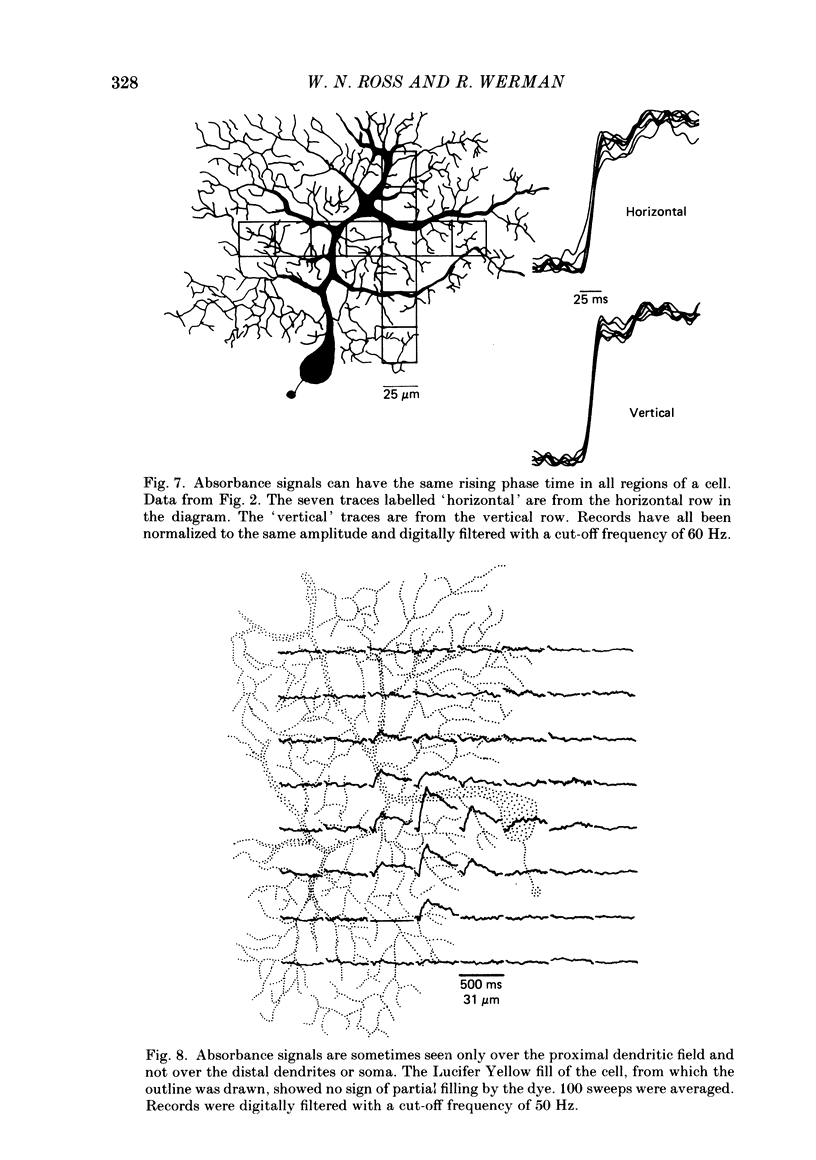




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Matteson D. R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985 Jan 4;227(4682):65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry into voltage-clamped presynaptic terminals of squid. J Physiol. 1985 Oct;367:143–162. doi: 10.1113/jphysiol.1985.sp015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Cohen L. B., De Weer P., Pinto L. H., Ross W. N., Salzberg B. M. Rapid changes in intracellular free calcium concentration. Detection by metallochromic indicator dyes in squid giant axon. Biophys J. 1975 Nov;15(11):1155–1160. doi: 10.1016/S0006-3495(75)85891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. Excitatory synaptic action in motoneurones. J Physiol. 1955 Nov 28;130(2):374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Ahmed Z. Diffusion of ions and indicator dyes in neural cytoplasm. Cell Mol Neurobiol. 1984 Mar;4(1):53–66. doi: 10.1007/BF00710942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Sears T. A. Effect of glutamate, aspartate and related derivatives on cerebellar purkinje cell dendrites in the rat: an in vitro study. J Physiol. 1982 Aug;329:297–317. doi: 10.1113/jphysiol.1982.sp014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986 Mar;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Intracellular calcium accumulation during depolarization in a molluscan neurone. J Physiol. 1980 Nov;308:259–285. doi: 10.1113/jphysiol.1980.sp013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K., Ross W. N. Regional distribution of calcium influx into bursting neurons detected with arsenazo III. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5565–5569. doi: 10.1073/pnas.82.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Cohen L. B., Lesher S., Boyle M. B. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia using a 124-element photodiode array. J Neurophysiol. 1981 May;45(5):829–840. doi: 10.1152/jn.1981.45.5.829. [DOI] [PubMed] [Google Scholar]
- Grinvald A. Real-time optical mapping of neuronal activity: from single growth cones to the intact mammalian brain. Annu Rev Neurosci. 1985;8:263–305. doi: 10.1146/annurev.ne.08.030185.001403. [DOI] [PubMed] [Google Scholar]
- Larramendi E. M., Victor T. Synapses on the Purkinje cell spines in the mouse. An electronmicroscopic study. Brain Res. 1967 May;5(1):15–30. doi: 10.1016/0006-8993(67)90216-8. [DOI] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Llinás R. Electroresponsive properties of dendrites in central neurons. Adv Neurol. 1975;12:1–13. [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Nicholson C., Precht W. Preferred centripetal conduction of dendritic spikes in alligator Purkinje cells. Science. 1969 Jan 10;163(3863):184–187. doi: 10.1126/science.163.3863.184. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Calcium transients recorded with arsenazo III in the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):197–211. doi: 10.1098/rspb.1981.0034. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Krauthamer V. Optical measurements of potential changes in axons and processes of neurons of a barnacle ganglion. J Neurosci. 1984 Mar;4(3):659–672. doi: 10.1523/JNEUROSCI.04-03-00659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Stockbridge L. L., Stockbridge N. L. Regional properties of calcium entry in barnacle neurons determined with Arsenazo III and a photodiode array. J Neurosci. 1986 Apr;6(4):1148–1159. doi: 10.1523/JNEUROSCI.06-04-01148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Stockbridge N., Ross W. N. Localized Ca2+ and calcium-activated potassium conductances in terminals of a barnacle photoreceptor. Nature. 1984 May 17;309(5965):266–268. doi: 10.1038/309266a0. [DOI] [PubMed] [Google Scholar]