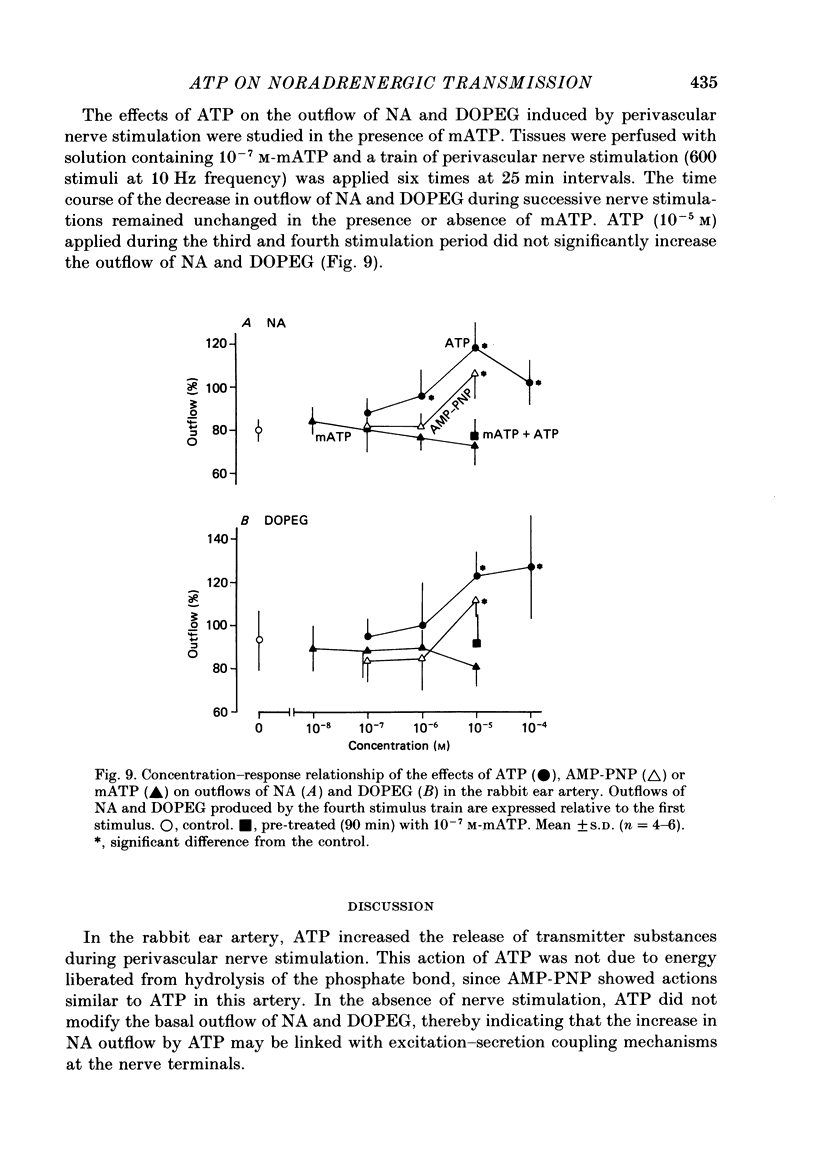
Abstract
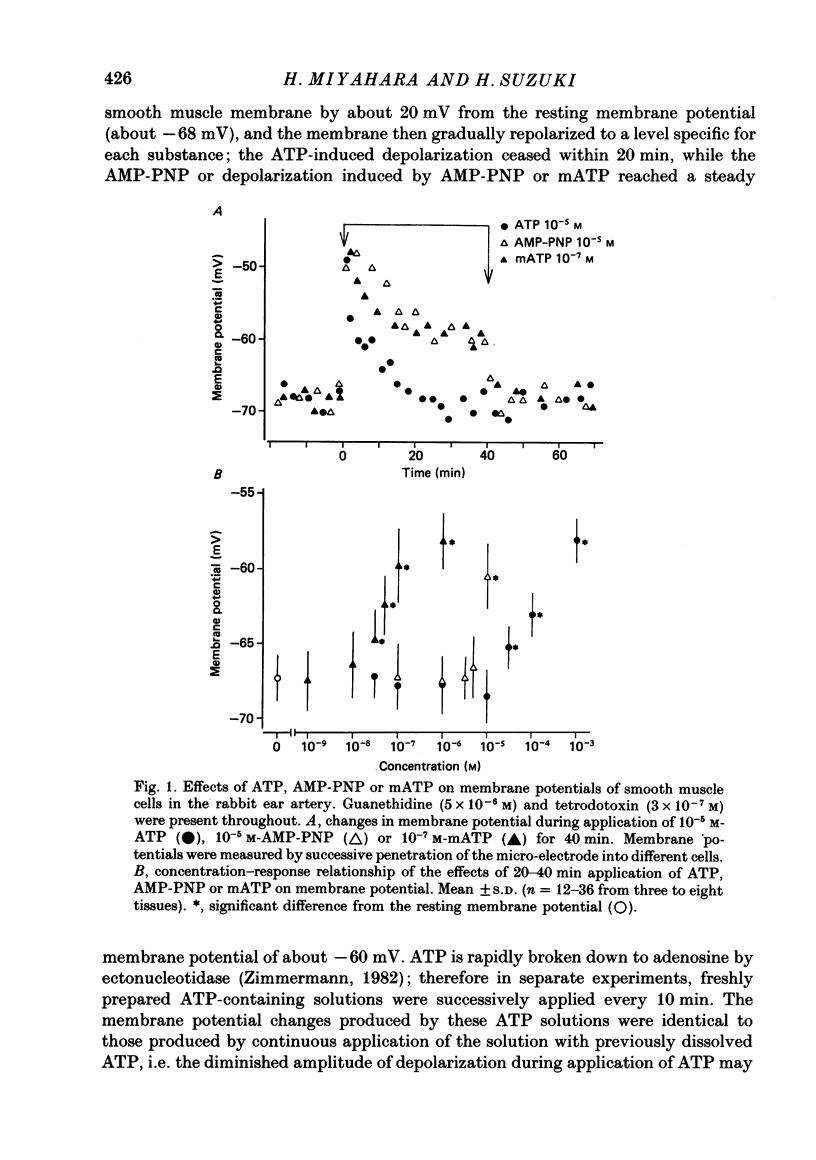
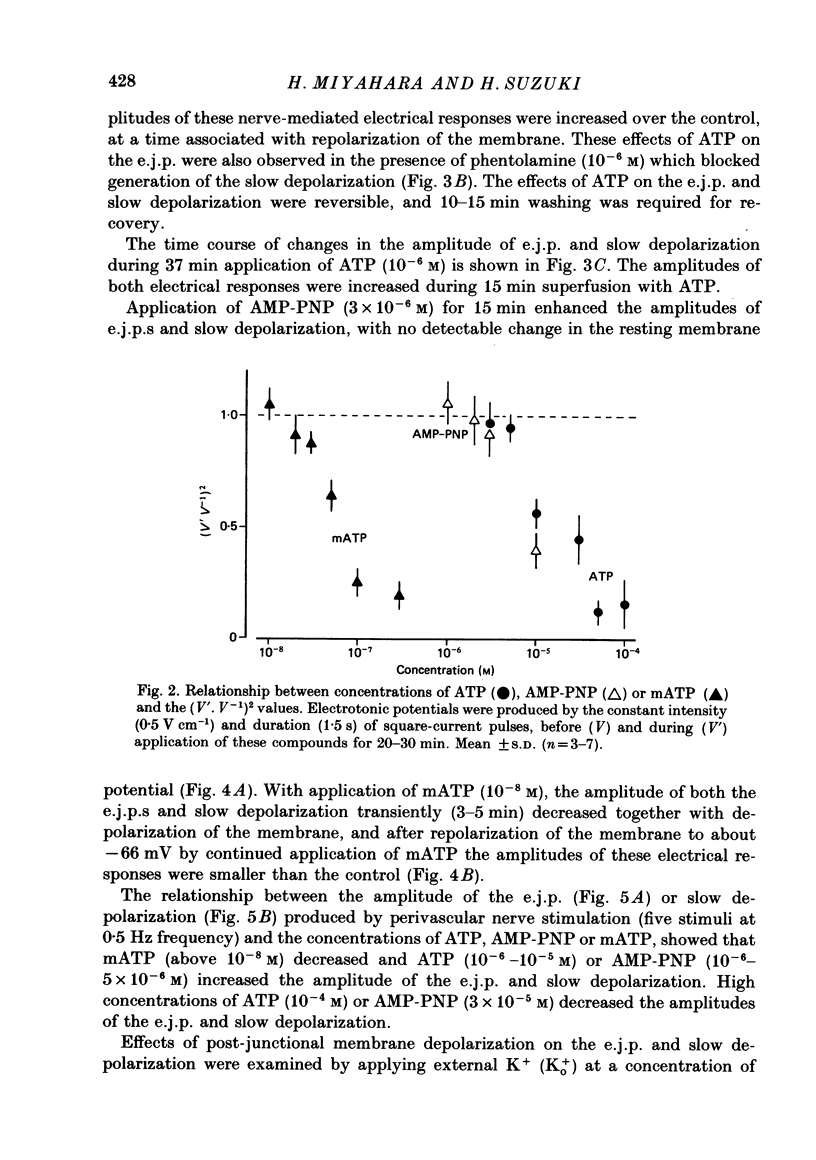
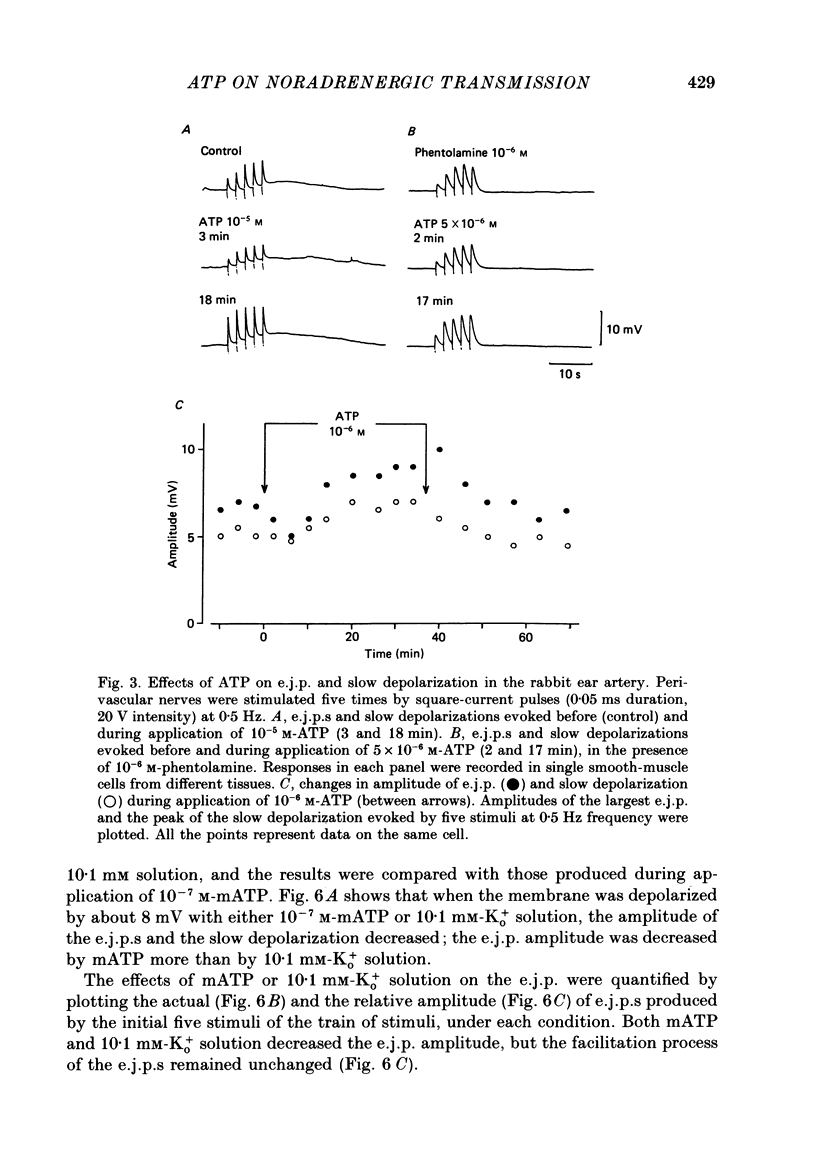
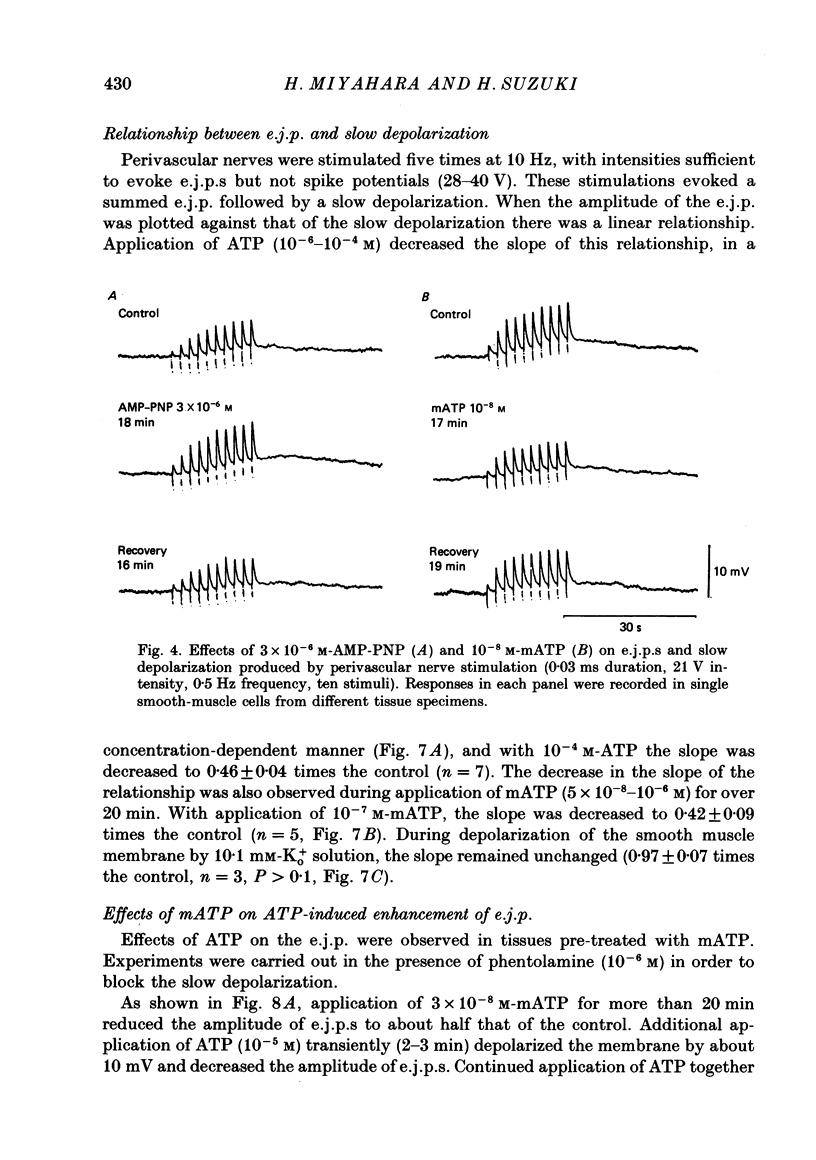
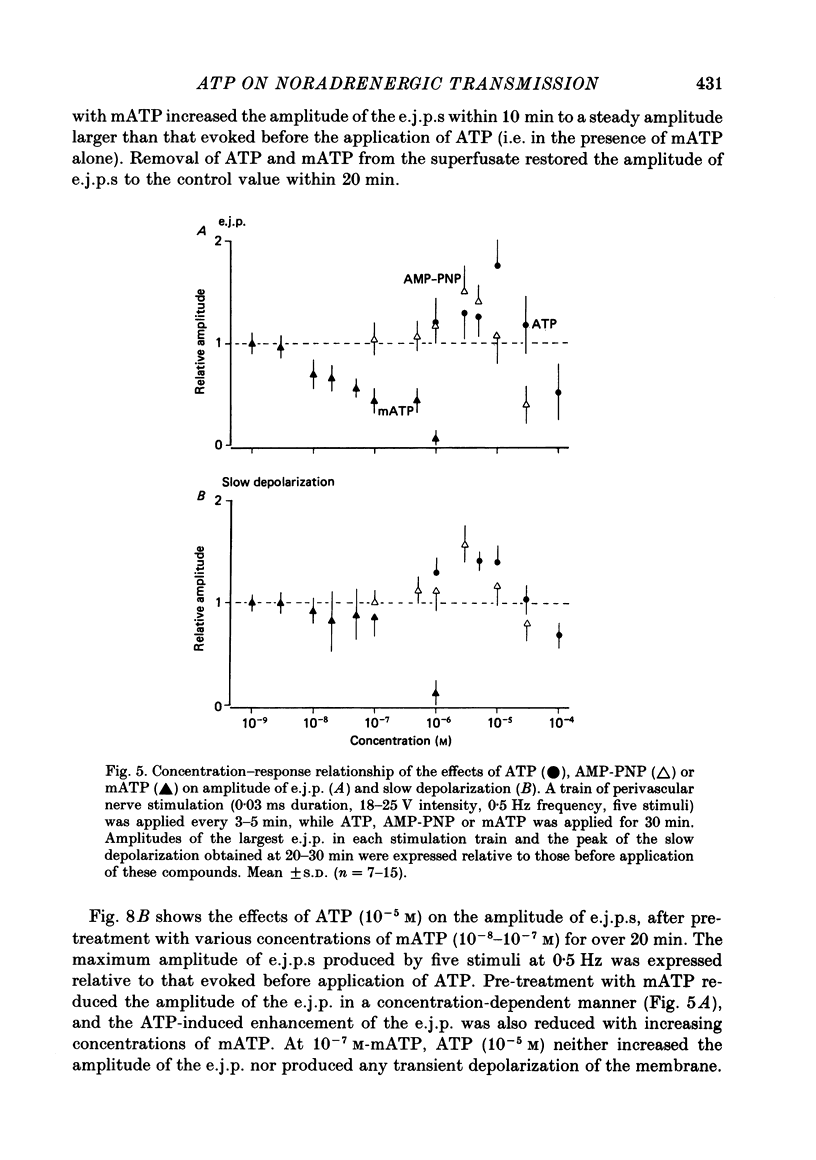
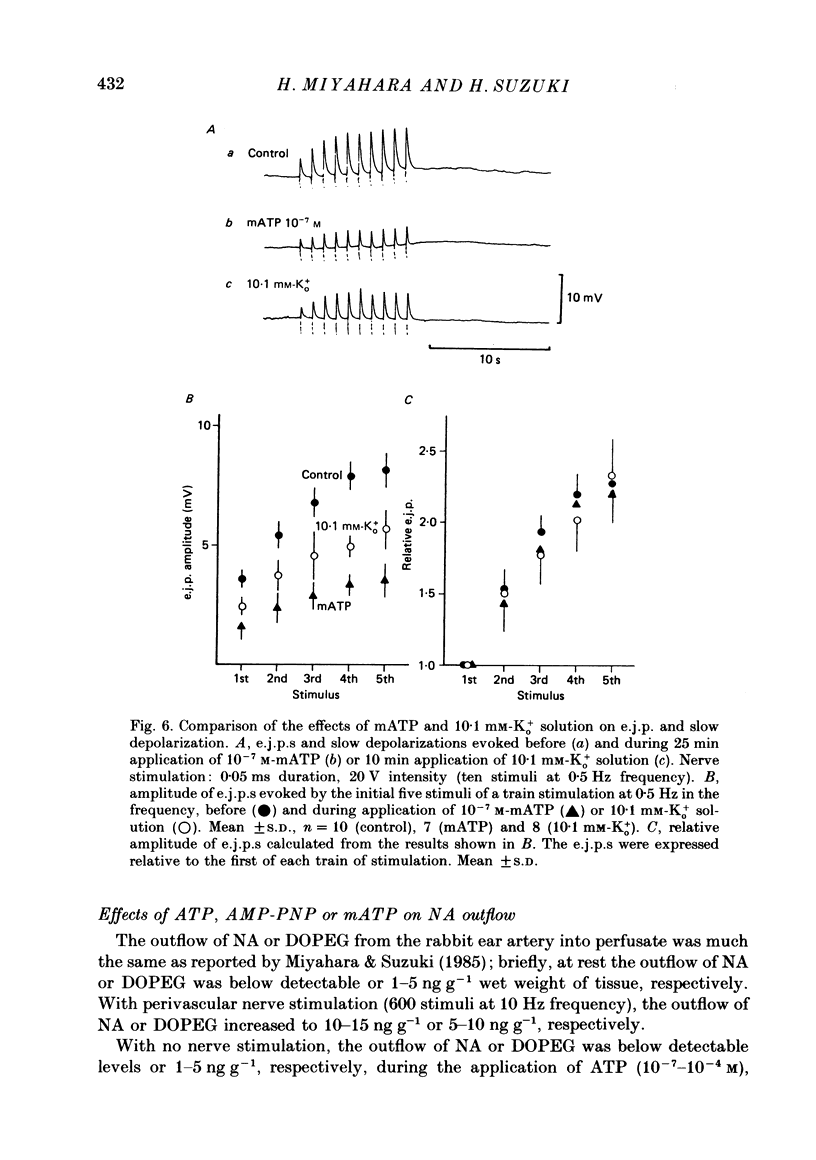
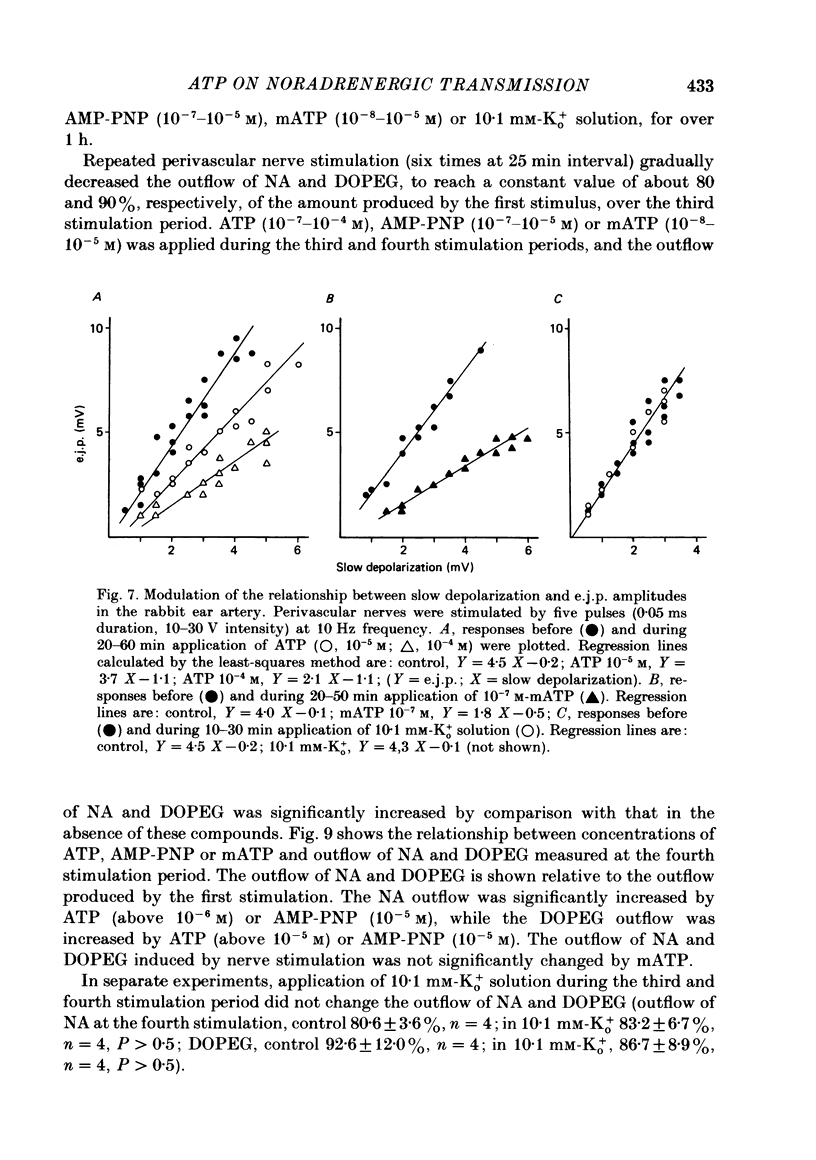
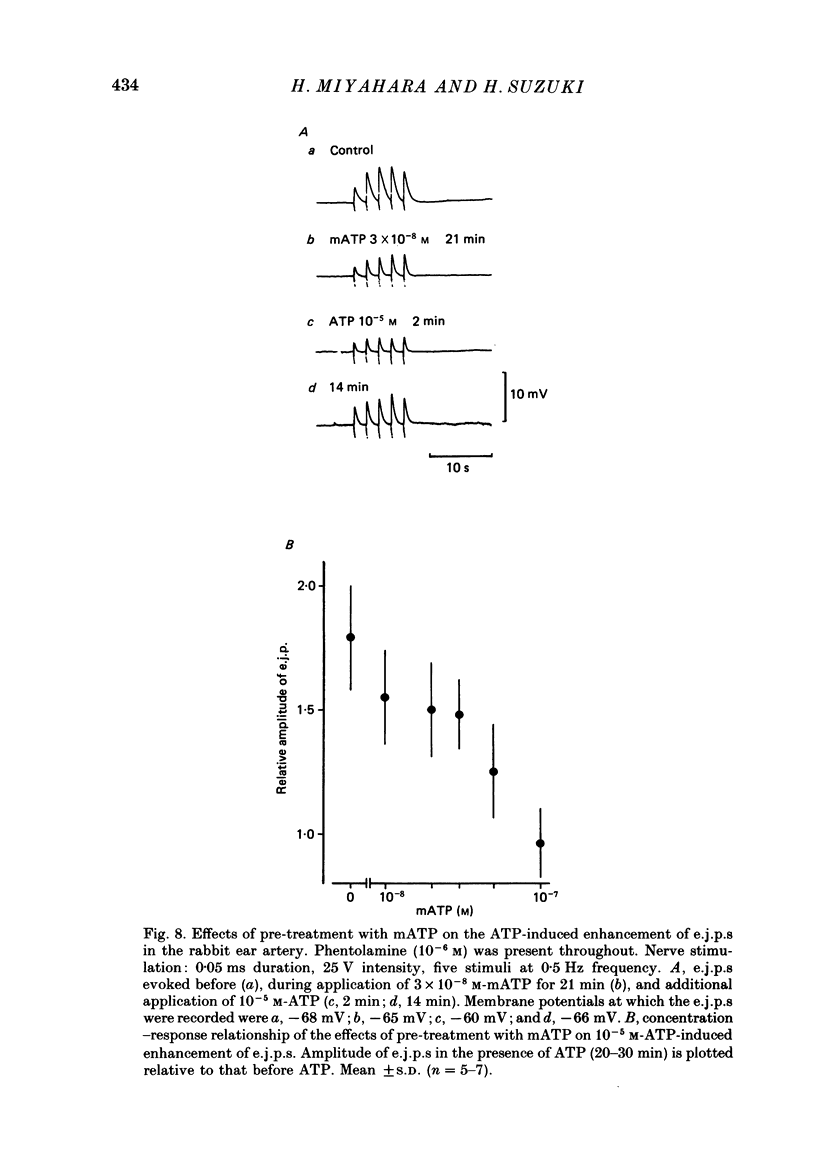
1. The effects of adenosine triphosphate (ATP), 5'-adenylylimidodiphosphate (AMP-PNP) or alpha,beta-methylene ATP (mATP) on the excitatory junction potential (e.j.p.) and slow depolarization evoked by perivascular nerve stimulation were studied in smooth muscle cells of the rabbit ear artery. 2. ATP (above 10(-6) M), AMP-PNP (above 10(-6) M) and mATP (above 10(-8) M) transiently (10-15 min) depolarized the membrane. The membrane remained depolarized after prolonged exposure (over 20 min) to ATP (above 3 X 10(-5) M), AMP-PNP (above 10(-5) M) or mATP (above 3 X 10(-8) M). 3. ATP (above 10(-5) M), AMP-PNP (above 5 X 10(-6) M) or mATP (above 3 X 10(-8) M) decreased the membrane resistance. Increasing the external K+ concentration (K+o) to 10.1 mM also decreased the membrane resistance, with an associated depolarization. 4. ATP (10(-6)-5 X 10(-5) M) or AMP-PNP (over 10(-6) M) transiently decreased and then increased amplitudes of the e.j.p. and of the slow depolarization, the latter component increasing more than the former. 5. Depolarization of the membrane by 10.1 mM-K+o solution or mATP (10(-7) M) decreased the amplitude of e.j.p.s, with no change in the facilitation, and the slope of the relationship between amplitude of e.j.p. and that of slow depolarization decreased with mATP but not with 10.1 mM-K+o solution. 6. The outflows of noradrenaline and 3,4-dihydroxyphenylglycol (DOPEG) induced by perivascular nerve stimulation increased with ATP (above 10(-6) M) or AMP-PNP (above 10(-5) M), while there was no change with mATP (10(-8)-10(-5) M) or 10.1 mM-K+o solution. 7. Pre-treatment with mATP inhibited the ATP-induced increase in the outflow of noradrenaline and DOPEG, and also the ATP-induced enhancement of the amplitude of the e.j.p. 8. Therefore ATP and AMP-PNP have predominantly excitatory actions on both pre- and post-junctional membranes, while mATP has an excitatory action on the post-junctional membrane but antagonizes the facilitatory action of ATP on release of noradrenaline from the nerve terminal.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. The effect of alpha, beta-methylene ATP on the depolarization evoked by noradrenaline (gamma-adrenoceptor response) and ATP in the immature rat basilar artery. Br J Pharmacol. 1986 May;88(1):6–8. doi: 10.1111/j.1476-5381.1986.tb09464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu L., Barnes E. M., Langer S. Z., Weiner N. Release of norepinephrine and dopamine-beta-hydroxylase by nerve stimulation. I. Role of neuronal and extraneuronal uptake and of alpha presynaptic receptors. J Pharmacol Exp Ther. 1974 Sep;190(3):431–450. [PubMed] [Google Scholar]
- Enero M. A., Saidman B. Q. Possible feed-back inhibition of noradrenaline release by purine compounds. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(1):39–46. doi: 10.1007/BF00508808. [DOI] [PubMed] [Google Scholar]
- Fujii K., Miyahara H., Suzuki H. Comparison of the effects of caffeine and procaine on noradrenergic transmission in the guinea-pig mesenteric artery. Br J Pharmacol. 1985 Mar;84(3):675–684. doi: 10.1111/j.1476-5381.1985.tb16149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Itoh T., Suzuki H. Membrane properties and excitatory neuromuscular transmission in the smooth muscle of dog cerebral arteries. Br J Pharmacol. 1982 Oct;77(2):197–208. doi: 10.1111/j.1476-5381.1982.tb09286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Hedlund H., Fändriks L., Delbro D., Fasth S. Blockade of non-cholinergic non-adrenergic colonic contraction in response to pelvic nerve stimulation by large doses of alpha, beta-methylene ATP. Acta Physiol Scand. 1983 Dec;119(4):451–454. doi: 10.1111/j.1748-1716.1983.tb07361.x. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., Fredholm B. B. Effects of adenosine on adrenergic neurotransmission; prejunctional inhibition and postjunctional enhancement. Naunyn Schmiedebergs Arch Pharmacol. 1976 Jun;293(3):217–223. doi: 10.1007/BF00507344. [DOI] [PubMed] [Google Scholar]
- Henseling M., Graefe K. H., Trendelenburg U. The rate constants for the efflux of the metabolites of noradrenaline from rabbit aortic strips. Naunyn Schmiedebergs Arch Pharmacol. 1978 Apr;302(2):207–215. doi: 10.1007/BF00517987. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978 Jul;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens W., Verhaeghe R. Modulation of the concentration of noradrenaline at the neuro-effector junction in human saphenous vein. Br J Pharmacol. 1983 Jun;79(2):577–585. doi: 10.1111/j.1476-5381.1983.tb11032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima T., Kuriyama H. Electrical properties of smooth muscle cell membrane and neuromuscular transmission in the guinea-pig basilar artery. Br J Pharmacol. 1981 Oct;74(2):495–504. doi: 10.1111/j.1476-5381.1981.tb09996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragi T., Su C. Purine release from vascular adrenergic nerves by high potassium and a calcium ionophore, A-23187. J Pharmacol Exp Ther. 1980 Dec;215(3):685–690. [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Adrenergic transmissions in the guinea-pig mesenteric artery and their cholinergic modulations. J Physiol. 1981 Aug;317:383–396. doi: 10.1113/jphysiol.1981.sp013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z., Pinto J. E. Possible involvement of a transmitter different from norepinephrine in the residual responses to nerve stimulation of the cat nictitating membrane after pretreatment with reserpine. J Pharmacol Exp Ther. 1976 Mar;196(3):697–713. [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Rectification in the smooth muscle cell membrane of rabbit aorta. J Physiol. 1976 Jun;258(2):269–278. doi: 10.1113/jphysiol.1976.sp011419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Mishima S., Miyahara H., Suzuki H. Transmitter release modulated by alpha-adrenoceptor antagonists in the rabbit mesenteric artery: a comparison between noradrenaline outflow and electrical activity. Br J Pharmacol. 1984 Oct;83(2):537–547. doi: 10.1111/j.1476-5381.1984.tb16518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara H., Suzuki H. Effects of tyramine on noradrenaline outflow and electrical responses induced by field stimulation in the perfused rabbit ear artery. Br J Pharmacol. 1985 Oct;86(2):405–416. doi: 10.1111/j.1476-5381.1985.tb08910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T., Suzuki H., Kuriyama H. Effects of flunarizine on electrical and mechanical responses of smooth muscle cells in basilar and ear arteries of the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1986 Aug;333(4):431–438. doi: 10.1007/BF00500020. [DOI] [PubMed] [Google Scholar]
- Oishi R., Mishima S., Kuriyama H. Determination of norepinephrine and its metabolites released from rat vas deferens using high-performance liquid chromatography with electrochemical detection. Life Sci. 1983 Feb 28;32(9):933–940. doi: 10.1016/0024-3205(83)90922-0. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Su C. Neurogenic release of purine compounds in blood vessels. J Pharmacol Exp Ther. 1975 Oct;195(1):159–166. [PubMed] [Google Scholar]
- Su C. Purinergic inhibition of adrenergic transmission in rabbit blood vessels. J Pharmacol Exp Ther. 1978 Feb;204(2):351–361. [PubMed] [Google Scholar]
- Su C., Tsuru H., Su M. O. Effects of adenosine triphosphate and prostaglandins on vascular adrenergic transmission. J Pharmacol Exp Ther. 1978 Oct;207(1):34–39. [PubMed] [Google Scholar]
- Suzuki H. An electrophysiological study of excitatory neuromuscular transmission in the guinea-pig main pulmonary artery. J Physiol. 1983 Mar;336:47–59. doi: 10.1113/jphysiol.1983.sp014565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Electrical responses of smooth muscle cells of the rabbit ear artery to adenosine triphosphate. J Physiol. 1985 Feb;359:401–415. doi: 10.1113/jphysiol.1985.sp015592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Fujiwara S. Neurogenic electrical responses of single smooth muscle cells of the dog middle cerebral artery. Circ Res. 1982 Dec;51(6):751–759. doi: 10.1161/01.res.51.6.751. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Verbeuren T. J., Webb R. C. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981 Jan;61(1):151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Hotta K. Electrical responses of the smooth muscle of the guinea-pig cerebral artery to brief electrical stimulation. Jpn J Physiol. 1986;36(1):77–90. doi: 10.2170/jjphysiol.36.77. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


