Abstract
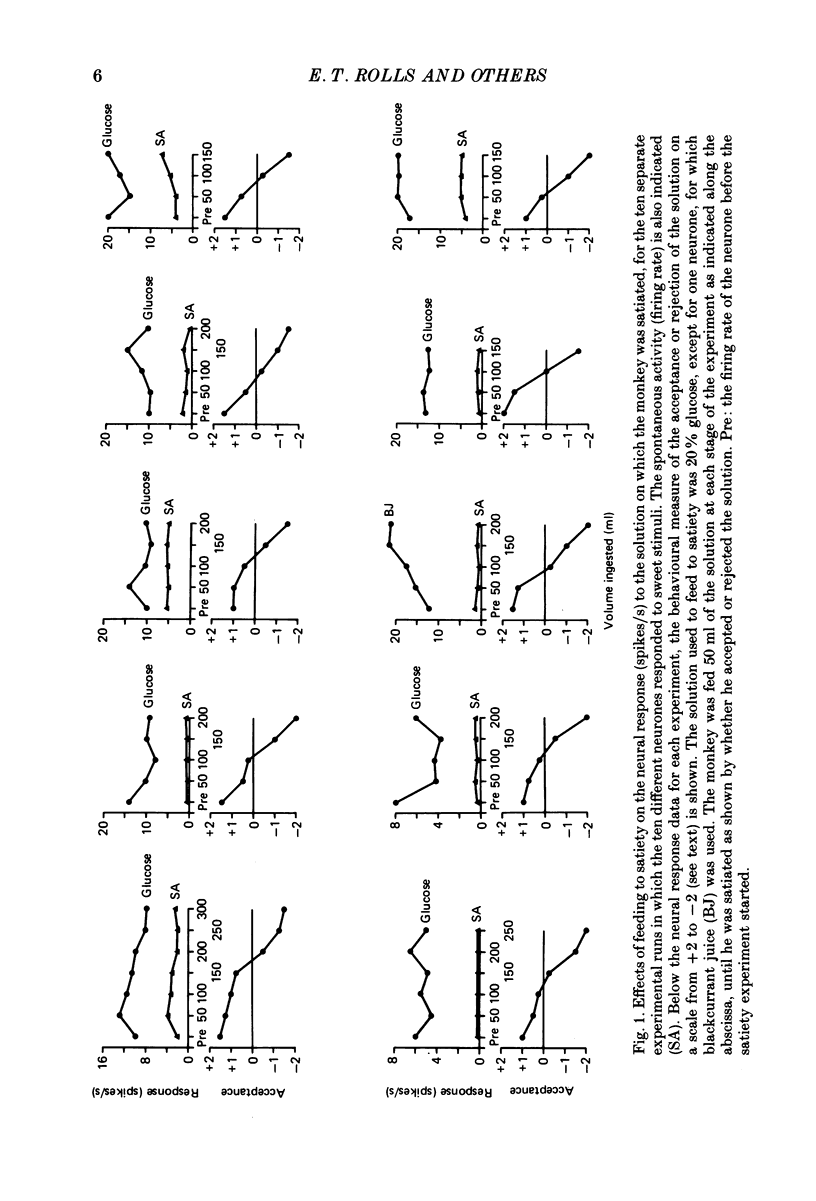
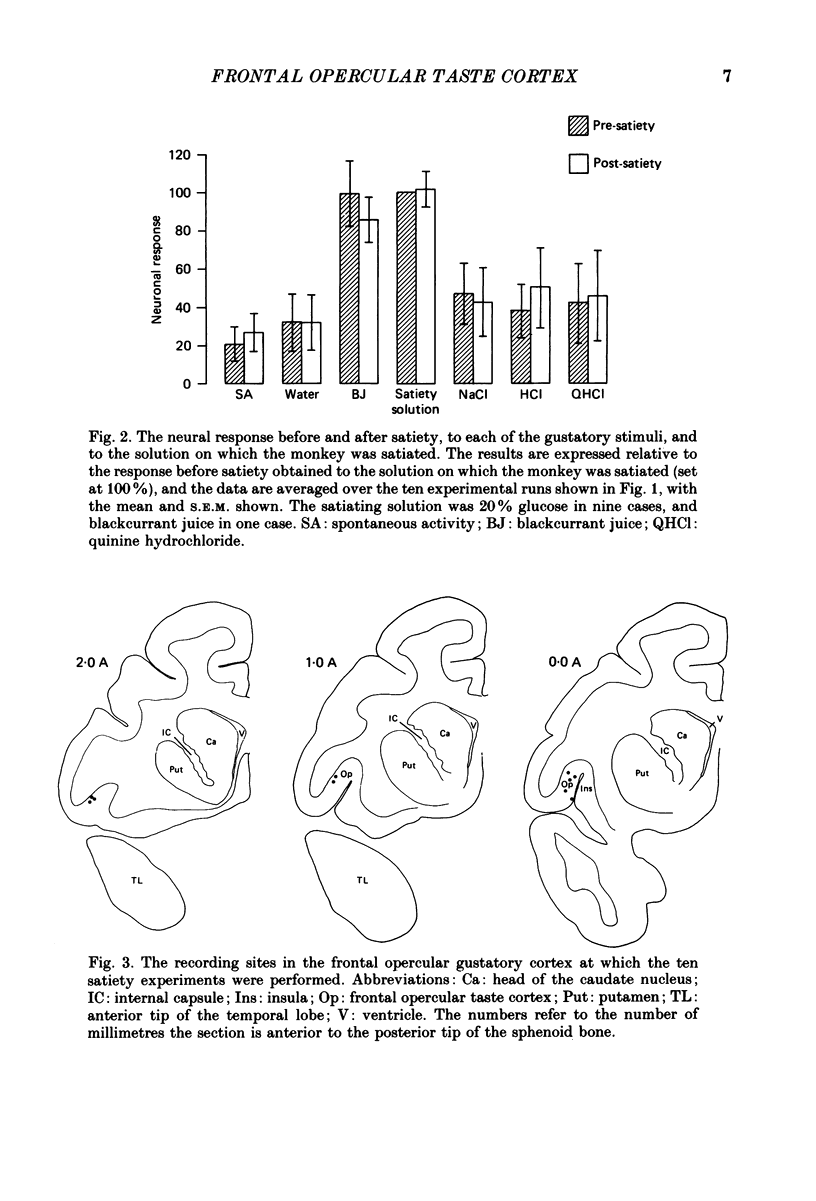
1. In order to determine whether the responsiveness of neurones in the primary gustatory cortex is influenced by hunger, the activity of neurones in the gustatory cortex in the frontal operculum was recorded while macaque monkeys (Macaca fascicularis) were fed to satiety. The responses of single neurones in the gustatory cortex to the prototypical taste stimuli glucose, NaCl, HCl and quinine hydrochloride, and to fruit juice, were measured before, while, and after the monkey was fed to satiety with glucose or fruit juice. 2. While behaviour turned from avid acceptance to active rejection upon repletion, the responsiveness of the neurones to the stimulus array, including the satiating solution, was unmodified. 3. It is concluded that in the gustatory cortex in the frontal operculum, neuronal responses to gustatory stimuli are not influenced by the normal transition from hunger to satiety. This is in contrast to the responses of a population of neurones recorded in the hypothalamus, which only occur to the taste of food when the monkey is hungry. Thus the neurones in the primary gustatory cortex are involved in a motivation-independent analysis of gustatory stimuli, whereas the hypothalamic neurones may be more closely related to the influence of motivational state on behavioural responsiveness to gustatory stimuli.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggleton J. P., Burton M. J., Passingham R. E. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res. 1980 May 26;190(2):347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- BAGSHAW M. H., PRIBRAM K. H. Cortical organization in gustation (Macaca mulatta). J Neurophysiol. 1953 Sep;16(5):499–508. doi: 10.1152/jn.1953.16.5.499. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M., Morse J. R., Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol. 1980 Mar 15;190(2):259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M., Norgren R. An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal, and vagal nerves in the monkey. J Comp Neurol. 1979 Apr 1;184(3):455–472. doi: 10.1002/cne.901840303. [DOI] [PubMed] [Google Scholar]
- Benjamin R. M., Burton H. Projection of taste nerve afferents to anterior opercular-insular cortex in squirrel monkey (Saimiri sciureus). Brain Res. 1968 Feb;7(2):221–231. doi: 10.1016/0006-8993(68)90100-5. [DOI] [PubMed] [Google Scholar]
- Burton M. J., Rolls E. T., Mora F. Effects of hunger on the responses of neurons in the lateral hypothalamus to the sight and taste of food. Exp Neurol. 1976 Jun;51(3):668–677. doi: 10.1016/0014-4886(76)90189-8. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971 Sep 17;173(4002):1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1(2):203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Giza B. K., Scott T. R. Blood glucose selectively affects taste-evoked activity in rat nucleus tractus solitarius. Physiol Behav. 1983 Nov;31(5):643–650. [PubMed] [Google Scholar]
- Gleen J. F., Erickson R. P. Gastric modulation of gustatory afferent activity. Physiol Behav. 1976 May;16(5):561–568. doi: 10.1016/0031-9384(76)90216-x. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982 Nov 20;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982 Nov 20;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Mesulam M. M. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982 Nov 20;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Mesulam M. M., Pandya D. N. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6(7):1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Pritchard T. C., Hamilton R. B., Morse J. R., Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986 Feb 8;244(2):213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Rolls B. J., Rolls E. T., Rowe E. A., Sweeney K. Sensory specific satiety in man. Physiol Behav. 1981 Jul;27(1):137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- Rolls B. J., Rowe E. A., Rolls E. T. How sensory properties of foods affect human feeding behavior. Physiol Behav. 1982 Sep;29(3):409–417. doi: 10.1016/0031-9384(82)90259-1. [DOI] [PubMed] [Google Scholar]
- Rolls B. J., Rowe E. A., Rolls E. T., Kingston B., Megson A., Gunary R. Variety in a meal enhances food intake in man. Physiol Behav. 1981 Feb;26(2):215–221. doi: 10.1016/0031-9384(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Rolls B. J., Van Duijvenvoorde P. M., Rolls E. T. Pleasantness changes and food intake in a varied four-course meal. Appetite. 1984 Dec;5(4):337–348. doi: 10.1016/s0195-6663(84)80006-9. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Burton M. J., Mora F. Hypothalamic neuronal responses associated with the sight of food. Brain Res. 1976 Jul 23;111(1):53–66. doi: 10.1016/0006-8993(76)91048-9. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Burton M. J., Mora F. Neurophysiological analysis of brain-stimulation reward in the monkey. Brain Res. 1980 Aug 4;194(2):339–357. doi: 10.1016/0006-8993(80)91216-0. [DOI] [PubMed] [Google Scholar]
- Rolls E. T. Central nervous mechanisms related to feeding and appetite. Br Med Bull. 1981 May;37(2):131–134. doi: 10.1093/oxfordjournals.bmb.a071689. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Judge S. J., Sanghera M. K. Activity of neurones in the inferotemporal cortex of the alert monkey. Brain Res. 1977 Jul 15;130(2):229–238. doi: 10.1016/0006-8993(77)90272-4. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Murzi E., Yaxley S., Thorpe S. J., Simpson S. J. Sensory-specific satiety: food-specific reduction in responsiveness of ventral forebrain neurons after feeding in the monkey. Brain Res. 1986 Mar 12;368(1):79–86. doi: 10.1016/0006-8993(86)91044-9. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Rolls B. J., Rowe E. A. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav. 1983 Feb;30(2):185–192. doi: 10.1016/0031-9384(83)90003-3. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Sanghera M. K., Roper-Hall A. The latency of activation of neurones in the lateral hypothalamus and substantia innominata during feeding in the monkey. Brain Res. 1979 Mar 23;164:121–135. doi: 10.1016/0006-8993(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Rolls E. T. The neurophysiology of feeding. Int J Obes. 1984;8 (Suppl 1):139–150. [PubMed] [Google Scholar]
- Russchen F. T., Amaral D. G., Price J. L. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol. 1985 Dec 1;242(1):1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Sanghera M. K., Rolls E. T., Roper-Hall A. Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Exp Neurol. 1979 Mar;63(3):610–626. doi: 10.1016/0014-4886(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Sanides F. The architecture of the cortical taste nerve areas in squirrel monkey (Saimiri sciureus) and their relationships to insular, sensorimotor and prefrontal regions. Brain Res. 1968 Apr;8(1):97–124. doi: 10.1016/0006-8993(68)90174-1. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Yaxley S., Sienkiewicz Z. J., Rolls E. T. Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol. 1986 Sep;56(3):876–890. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Yaxley S., Sienkiewicz Z. J., Rolls E. T. Gustatory responses in the nucleus tractus solitarius of the alert cynomolgus monkey. J Neurophysiol. 1986 Jan;55(1):182–200. doi: 10.1152/jn.1986.55.1.182. [DOI] [PubMed] [Google Scholar]
- Sudakov K., MacLean P. D., Reeves A., Marino R. Unit study of exteroceptive inputs to claustrocortex in awake, sitting, squirrel monkey. Brain Res. 1971 Apr 16;28(1):19–34. doi: 10.1016/0006-8993(71)90521-x. [DOI] [PubMed] [Google Scholar]
- Yaxley S., Rolls E. T., Sienkiewicz Z. J., Scott T. R. Satiety does not affect gustatory activity in the nucleus of the solitary tract of the alert monkey. Brain Res. 1985 Nov 11;347(1):85–93. doi: 10.1016/0006-8993(85)90891-1. [DOI] [PubMed] [Google Scholar]



