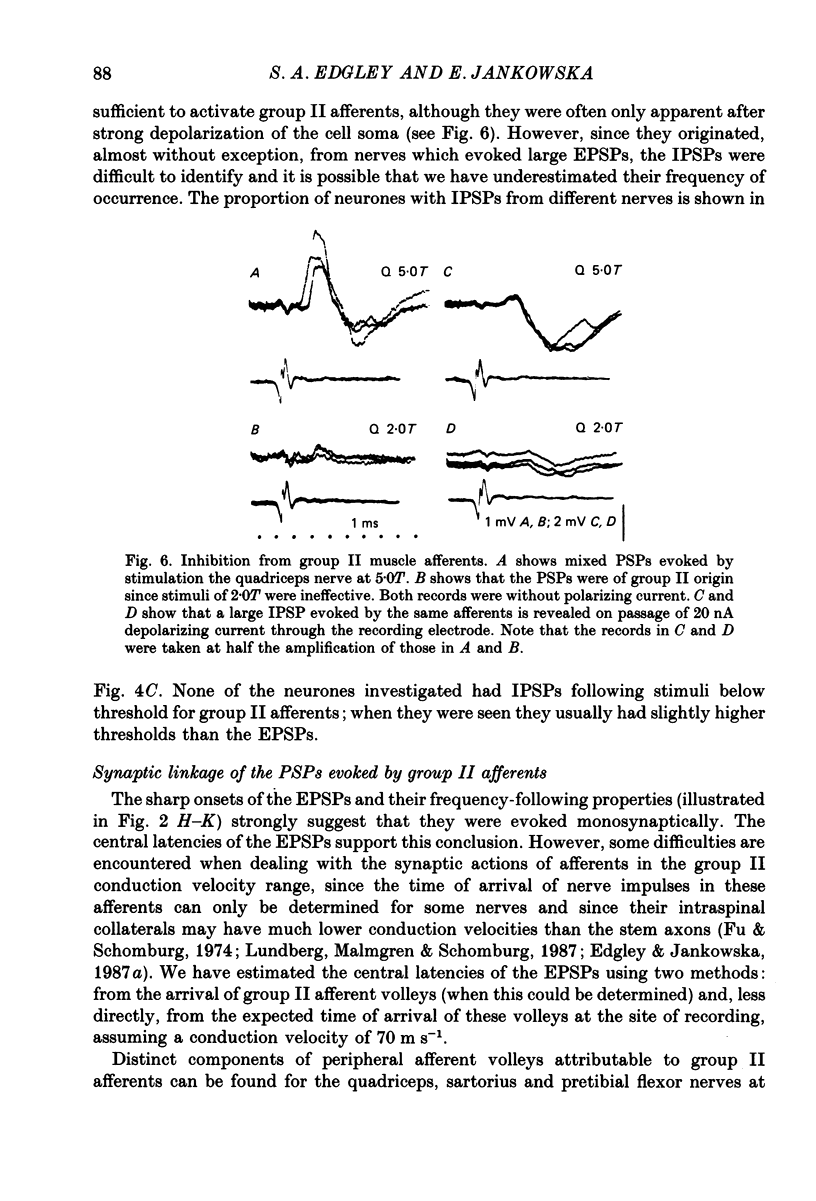
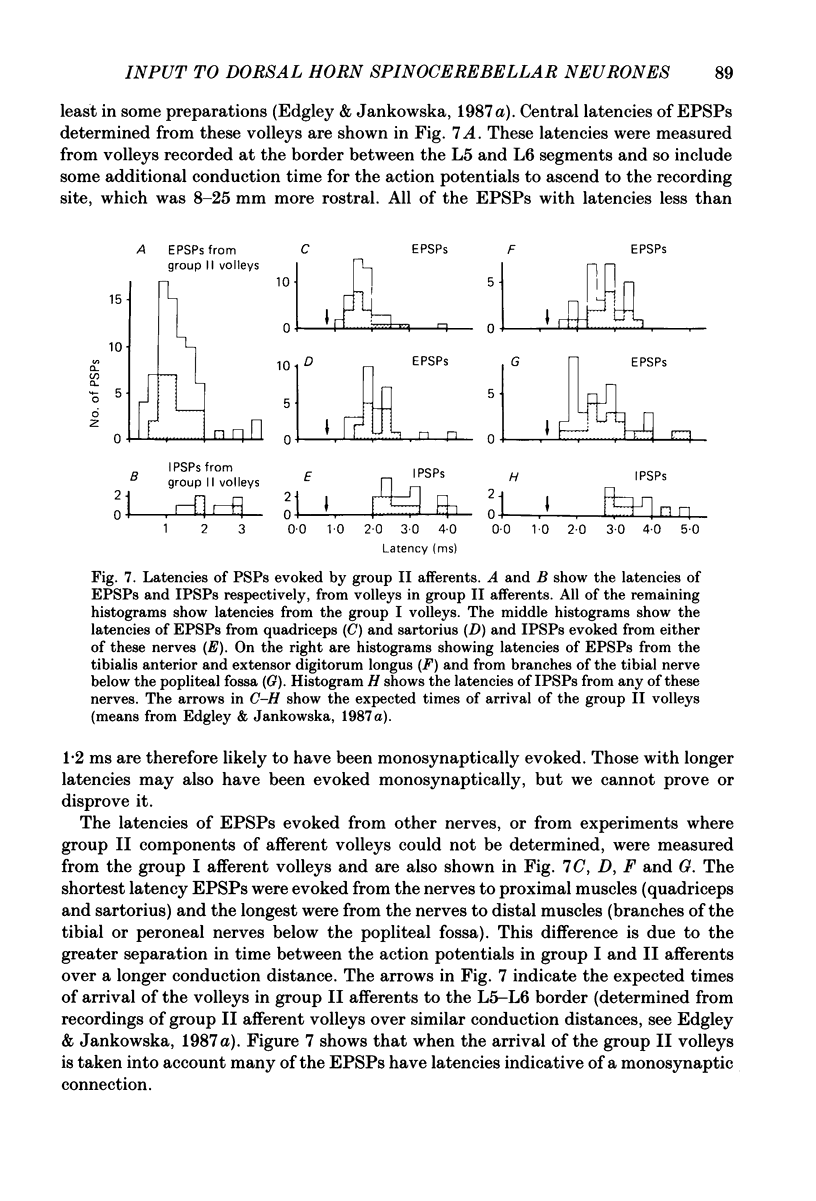
Abstract
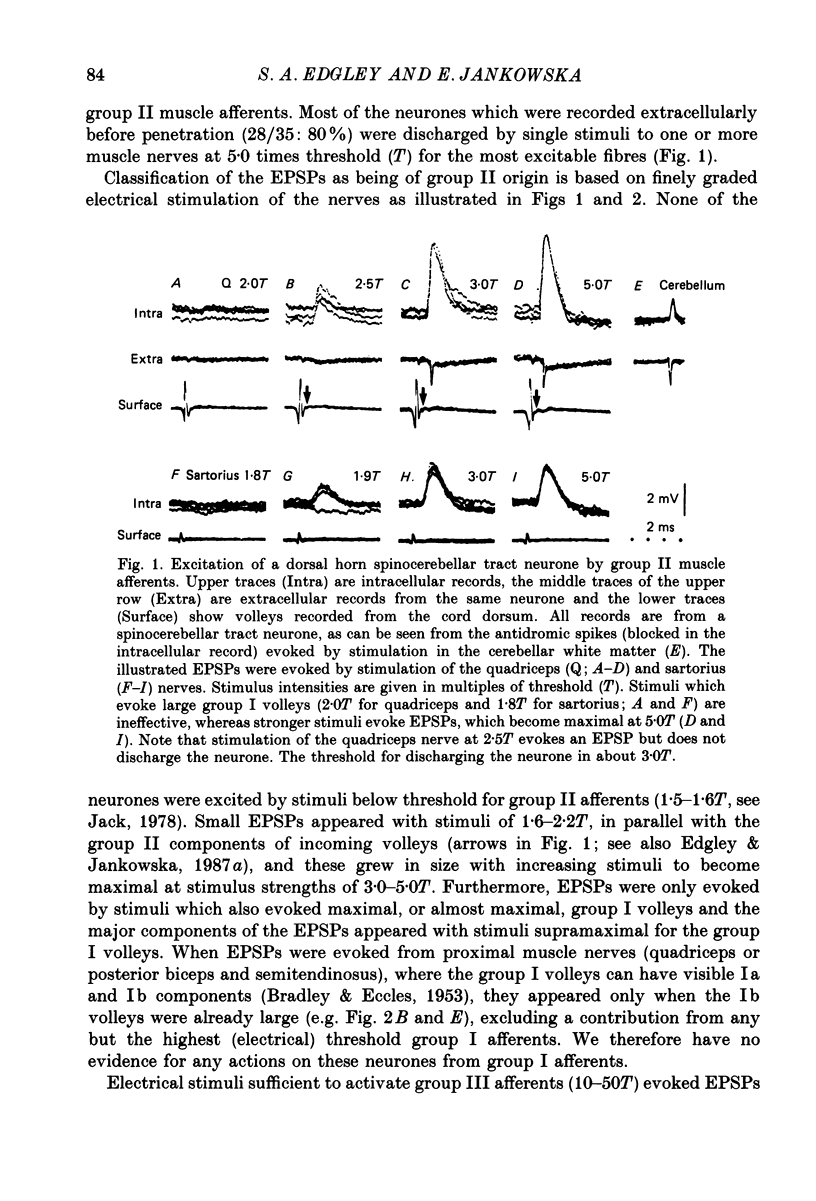
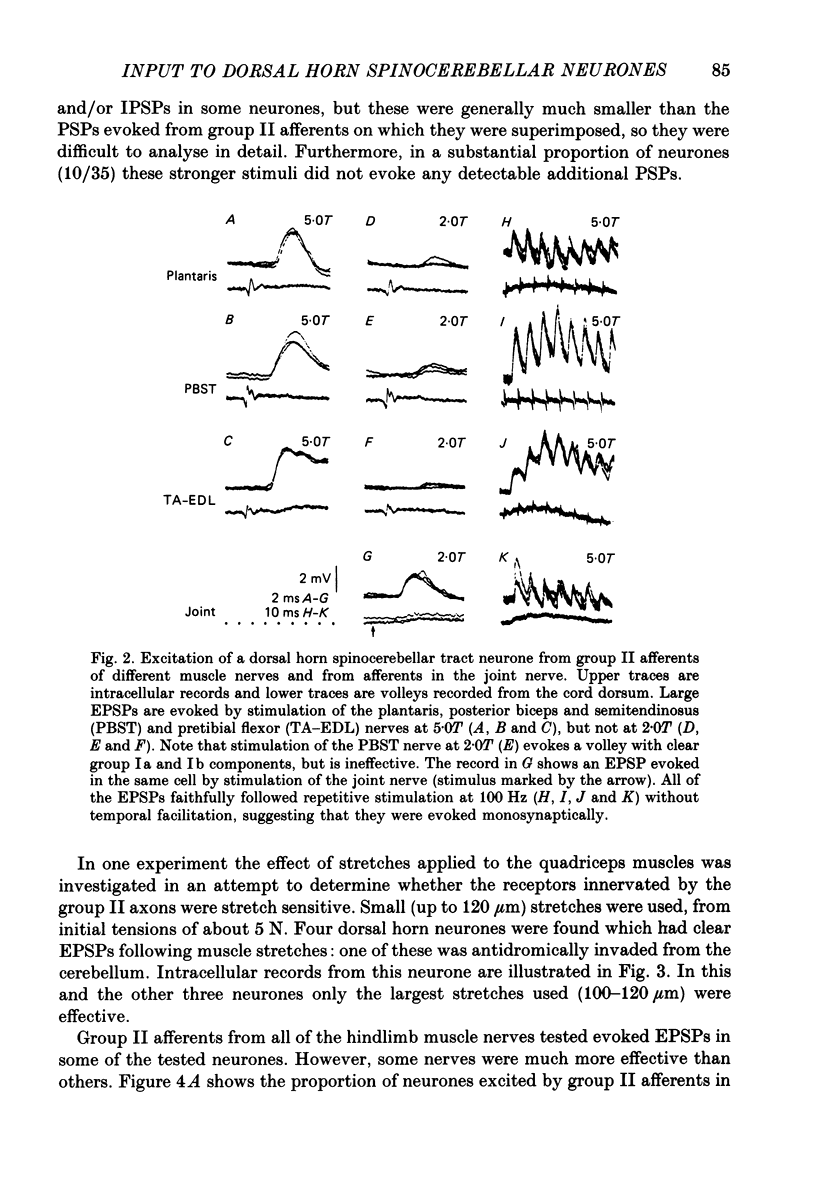
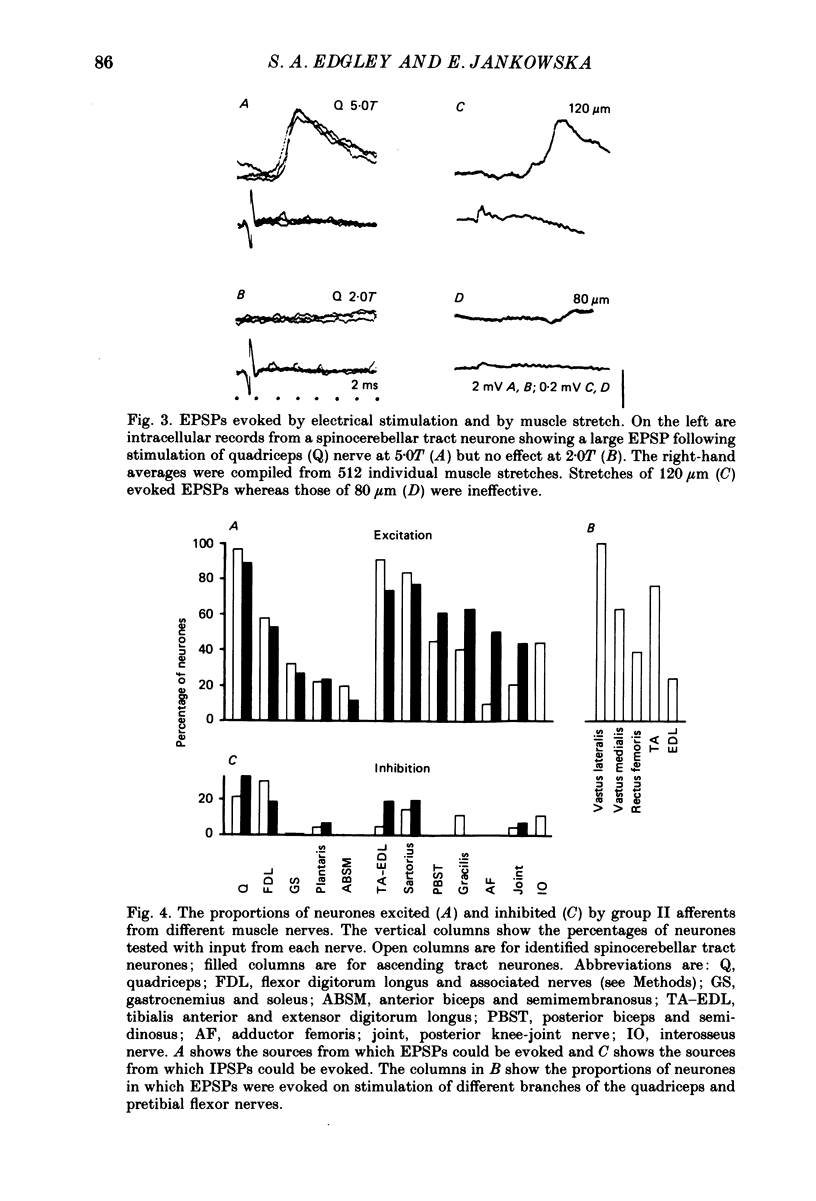
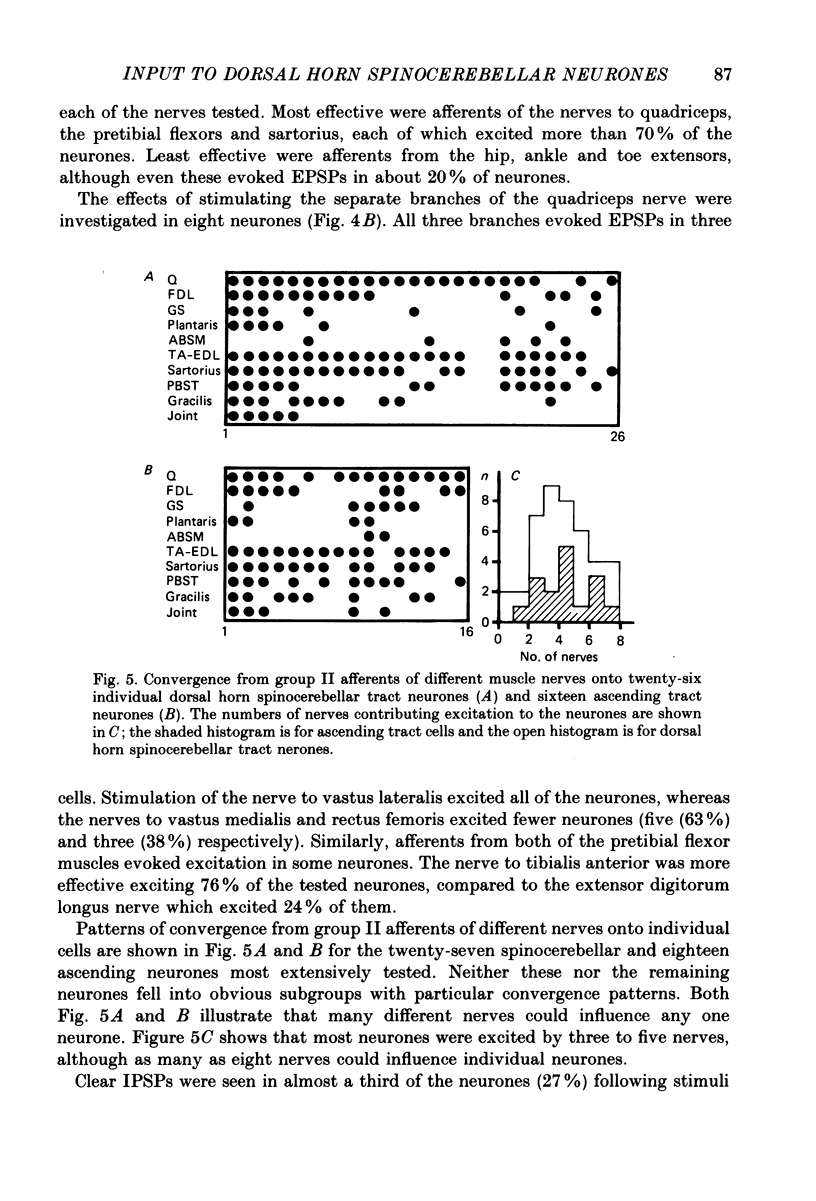
1. A group of spinocerebellar tract neurones located in the dorsal horn of the mid-lumbar segments of the spinal cord but outside of Clarke's column have been investigated by intracellular and extracellular recording from their somata. The existence of these neurones has been demonstrated previously using anatomical methods, but their properties have not been investigated in detail. In contrast to the cells of Clarke's column, these neurones were found to process information from both exteroceptors and proprioceptors. 2. All of the investigated neurones were powerfully excited following stimulation of muscle nerves at strengths sufficient to activate group II afferents while there was no evidence for actions from group I afferents onto any of them. Most were excited by group II afferents from many different nerves, including those from muscles acting on different joints. The latencies and properties of the excitatory postsynaptic potentials (EPSPs) suggest that at least a large proportion of them were monosynaptically evoked. 3. All of the neurones were powerfully excited following electrical stimulation of cutaneous afferents. The most potent effects were evoked from the saphenous and sural nerves which innervate the skin of the leg and thigh. In many cases these EPSPs had latencies indicative of a monosynaptic connection. The superficial peroneal and tibial nerves which innervate the skin of the foot evoked EPSPs which were usually smaller and of longer latency. 4. Responses to adequate stimulation of cutaneous afferents were examined in twenty extracellularly recorded neurones. All but one of them could be discharged by weak mechanical stimulation of the skin over the proximal part of the leg and thigh. None were activated from the skin of the foot. 5. Some of the neurones were influenced by stimulation of the posterior knee joint or interosseous nerves. These actions were relatively weak, however, suggesting that the powerful effects seen on stimulation of muscle nerves were unlikely to have been mediated by articular or Pacinian afferents which contaminate them. 6. Excitation from group II afferents was sometimes followed by inhibition (in 27% of the neurones). In almost all cases the inhibitory postsynaptic potentials (IPSPs) were evoked from the same nerves which evoked EPSPs. The minimal latencies of the IPSPs were approximately 1.0 ms longer than those of the EPSPs, suggesting that they were evoked disynaptically. 7. The possibility that these neurones provide information regarding limb position is discussed.
Full text
PDF



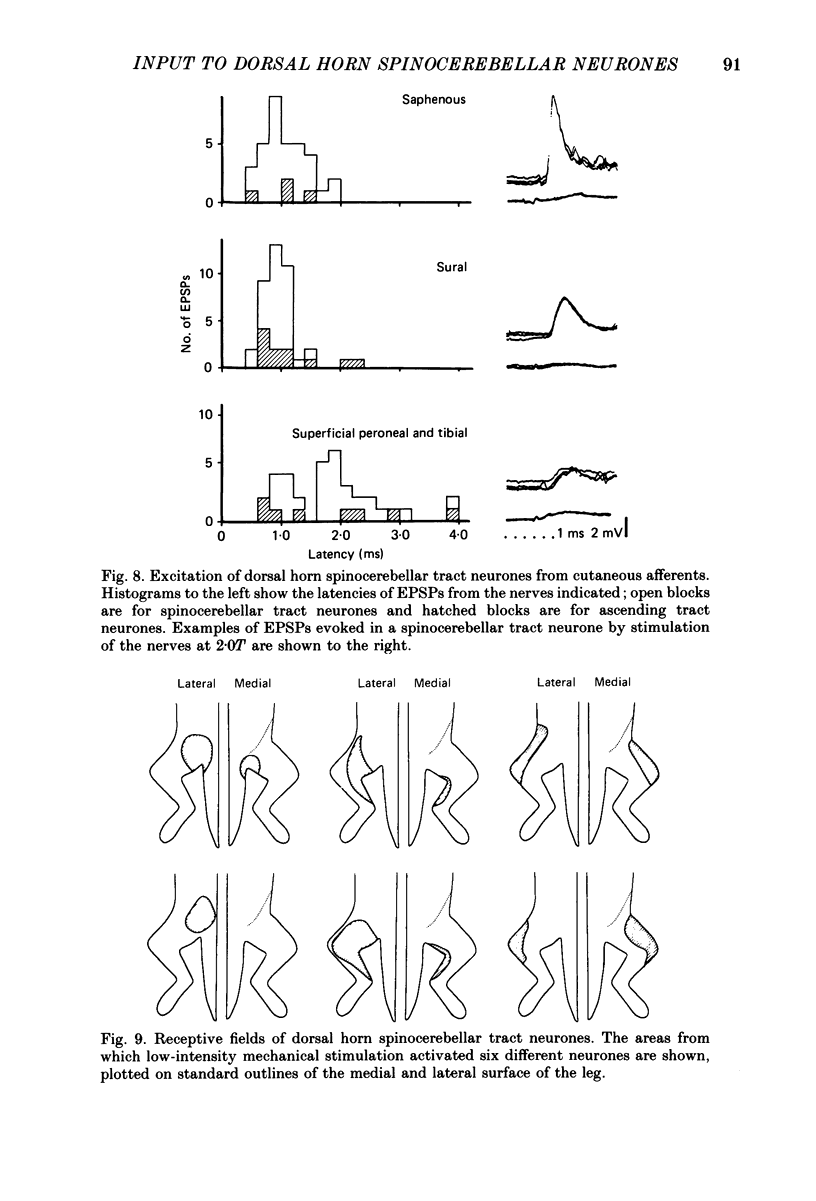






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama M., Hongo T., Kudo N. An uncrossed ascending tract originating from below Clarke's column and conveying group I impulses from the hindlimb muscles in the cat. Brain Res. 1973 Nov 9;62(1):237–241. doi: 10.1016/0006-8993(73)90634-3. [DOI] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. The spinocervical tract. Prog Neurobiol. 1981;17(1-2):59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Wei J. Y., Clark F. J., Simon J. Signaling of kinesthetic information by peripheral sensory receptors. Annu Rev Neurosci. 1982;5:171–187. doi: 10.1146/annurev.ne.05.030182.001131. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Gallimore C. M. The morphology and projections of dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988 Mar;397:99–111. doi: 10.1113/jphysiol.1988.sp016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987 Aug;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Gordon G., Grant G. Dorsolateral spinal afferents to some medullary sensory nuclei. An anatomical study in the cat. Exp Brain Res. 1982;46(1):12–23. doi: 10.1007/BF00238093. [DOI] [PubMed] [Google Scholar]
- Grant G., Wiksten B., Berkley K. J., Aldskogius H. The location of cerebellar-projecting neurons within the lumbosacral spinal cord in the cat. An anatomical study with HRP and retrograde chromatolysis. J Comp Neurol. 1982 Feb 1;204(4):336–348. doi: 10.1002/cne.902040405. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Ohno T., Sasaki S., Yamashita M., Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983 Sep;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Rudjord T. Dorsal spinocerebellar tract: response pattern of nerve fibers to muscle stretch. Science. 1965 Sep 3;149(3688):1109–1111. doi: 10.1126/science.149.3688.1109. [DOI] [PubMed] [Google Scholar]
- Johansson H., Silfvenius H. Axon-collateral activation by dorsal spinocerebellar tract fibres of group I relay cells of nucleus Z in the cat medulla oblongata. J Physiol. 1977 Feb;265(2):341–369. doi: 10.1113/jphysiol.1977.sp011720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Muñoz-Martinez E. J., Randić M. Sensory inputs to neurones in Clarke's column from muscle, cutaneous and joint receptors. J Physiol. 1973 Jan;228(2):327–342. doi: 10.1113/jphysiol.1973.sp010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. II. Single fibre recording in Flechsig's fasciculus on electrical stimulation of various peripheral nerves. Acta Physiol Scand. 1956 Mar 24;36(1-2):188–203. doi: 10.1111/j.1748-1716.1956.tb01317.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. VII. Identification of units by antidromic activation from the cerebellar cortex with recognition of five functional subdivisions. Acta Physiol Scand. 1960 Dec 30;50:356–374. doi: 10.1111/j.1748-1716.1960.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Landgren S., Silfvenius H. Nucleus Z, the medullary relay in the projection path to the cerebral cortex of group I muscle afferents from the cat's hind limb. J Physiol. 1971 Nov;218(3):551–571. doi: 10.1113/jphysiol.1971.sp009633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12(3):317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987;65(2):271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12(3):295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- Magherini P. C., Pompeiano O., Seguin J. J. Responses of nucleus z neurons to vibration of hindlimb extensor muscles in the decerebrate cat. Arch Ital Biol. 1975 Jun;113(2):150–187. [PubMed] [Google Scholar]
- Matsushita M., Hosoya Y., Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979 Mar 1;184(1):81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Where does Sherrington's "muscular sense" originate? Muscles, joints, corollary discharges? Annu Rev Neurosci. 1982;5:189–218. doi: 10.1146/annurev.ne.05.030182.001201. [DOI] [PubMed] [Google Scholar]
- McIntyre A. K., Proske U., Rawson J. A. Pathway to the cerebral cortex for impulses from tendon organs in the cat's hind limb. J Physiol. 1985 Dec;369:115–126. doi: 10.1113/jphysiol.1985.sp015891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar I., Kuypers H. G. Cells of origin of propriospinal fibers and of fibers ascending to supraspinal levels. A HRP study in cat and rhesus monkey. Brain Res. 1978 Sep 8;152(3):429–450. doi: 10.1016/0006-8993(78)91102-2. [DOI] [PubMed] [Google Scholar]
- Randić M., Myslinski N. R., Gordon J. H. Spinal localization of neurons receiving inputs from cutaneous afferents in the cat hindlimb. Brain Res. 1976 Apr 9;105(3):573–577. doi: 10.1016/0006-8993(76)90606-5. [DOI] [PubMed] [Google Scholar]
- Rustioni A., Kaufman A. B. Identification of cells or origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res. 1977 Jan 18;27(1):1–14. doi: 10.1007/BF00234821. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Mann M. D., Brown P. B., Cogdell B. Cells of origin of the cutaneous subdivision of the dorsal spinocerebellar tract. Brain Res. 1975 Feb 21;85(1):59–63. doi: 10.1016/0006-8993(75)91005-7. [DOI] [PubMed] [Google Scholar]
- Wei J. Y., Simon J., Randić M., Burgess P. R. Ascending spinal axons that signal the position of the hindlimbs under static conditions: location and receptor input. Exp Brain Res. 1984;54(1):7–22. doi: 10.1007/BF00235814. [DOI] [PubMed] [Google Scholar]



