Abstract
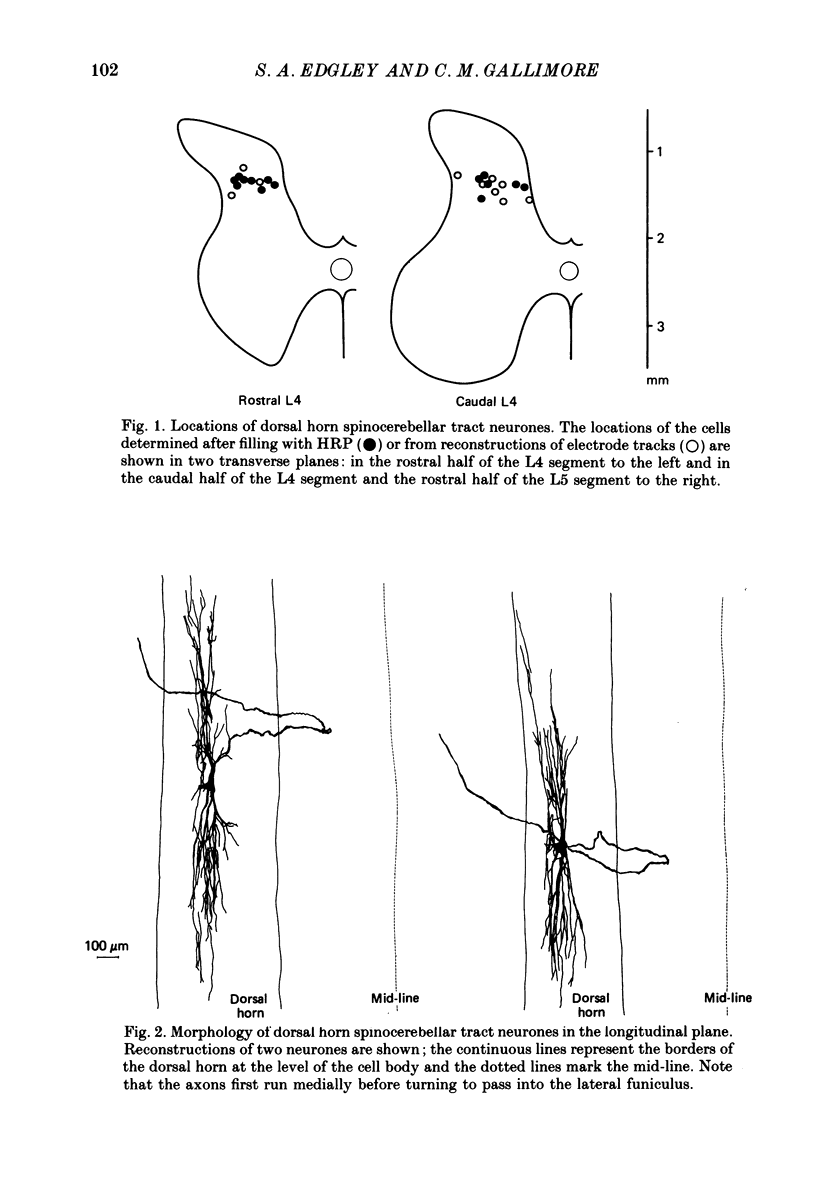
1. The morphology of dorsal horn neurones located in the mid-lumbar segments of the spinal cord and which have an axonal projection to the cerebellum has been investigated. The neurones were identified by antidromic activation from the cerebellum and by their characteristic input from group II afferents as described in the preceding paper (Edgley & Jankowska, 1988). 2. The cell bodies of the neurones were distributed across the width of the spinal cord in laminae IV and V, but particularly at the border between these laminae. Most were in the caudal half of the fourth lumbar segment (L4), caudal to Clarke's column. However, neurones of this type were encountered as far caudal as the middle of the fifth lumbar segment (L5) and as far rostral as the middle of the third lumbar segment (L3). 3. The morphology of the neurones was investigated following intracellular staining with horseradish peroxidase (HRP). Fourteen well-filled cells were recovered. They had large somata and extensive dendritic arborizations within the dorsal horn which could extend more than 2 mm rostro-caudally. The most dense arborization was in laminae III and IV, just dorsal to the cell bodies. 4. The axons of all fourteen cells could be followed well into the white matter. All of them passed into the dorsal part of the ipsilateral lateral funiculus where they ascended. All followed a similar indirect course through the grey matter. Despite careful inspection, initial axon collaterals were never found. 5. All of the neurones were antidromically activated by low-intensity electrical stimulation of the dorsolateral part of the ipsilateral lateral funiculus in the thoracic region and from the cerebellum. The conduction velocities of the axons ranged from 62 to 112 m s-1 (mean 84.2 (S.D. +/- 10.1) m s-1). 6. The axonal terminations of some neurones were investigated by mapping the most effective locations for antidromic activation from the cerebellar cortex. Most neurones were activated with lowest stimulus intensities from the rostral part of the anterior lobe. A second effective area was found in the posterior lobe, deep to the paramedian lobule. The majority of neurones were activated from both locations, suggesting that their axons branched to terminate in both areas. 7. On the basis of their projection and termination, it is proposed that the axons of these dorsal horn spinocerebellar tract neurones contribute to the dorsal spinocerebellar tract (DSCT).
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama M., Hongo T., Kudo N. An uncrossed ascending tract originating from below Clarke's column and conveying group I impulses from the hindlimb muscles in the cat. Brain Res. 1973 Nov 9;62(1):237–241. doi: 10.1016/0006-8993(73)90634-3. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rose P. K., Snow P. J. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:409–428. doi: 10.1113/jphysiol.1980.sp013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977 Nov;272(3):779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones revealed by intracellular injection of horseradish peroxidase. J Physiol. 1977 Sep;270(3):747–764. doi: 10.1113/jphysiol.1977.sp011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. The spinocervical tract. Prog Neurobiol. 1981;17(1-2):59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988 Mar;397:81–97. doi: 10.1113/jphysiol.1988.sp016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G., Wiksten B., Berkley K. J., Aldskogius H. The location of cerebellar-projecting neurons within the lumbosacral spinal cord in the cat. An anatomical study with HRP and retrograde chromatolysis. J Comp Neurol. 1982 Feb 1;204(4):336–348. doi: 10.1002/cne.902040405. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hongo T., Okada Y., Sato M. Corticofugal influences on transmission to the dorsal spinocerebellar tract from hindlimb primary afferents. Exp Brain Res. 1967;3(2):135–149. doi: 10.1007/BF00233258. [DOI] [PubMed] [Google Scholar]
- Houchin J., Maxwell D. J., Fyffe R. E., Brown A. G. Light and electron microscopy of dorsal spinocerebellar tract neurones in the cat: an intracellular horseradish peroxidase study. Q J Exp Physiol. 1983 Oct;68(4):719–732. doi: 10.1113/expphysiol.1983.sp002761. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Westman J. Intracellular application of horseradish peroxidase and its light and electron microscopical appearance in spinocervical tract cells. Brain Res. 1976 Apr 9;105(3):557–562. doi: 10.1016/0006-8993(76)90603-x. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. D. Clarke's column and the dorsal spinocerebellar tract: a review. Brain Behav Evol. 1973;7(1):34–83. doi: 10.1159/000124397. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Hosoya Y., Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979 Mar 1;184(1):81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- Molenaar I., Kuypers H. G. Cells of origin of propriospinal fibers and of fibers ascending to supraspinal levels. A HRP study in cat and rhesus monkey. Brain Res. 1978 Sep 8;152(3):429–450. doi: 10.1016/0006-8993(78)91102-2. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Randić M., Miletić V., Loewy A. D. A morphological study of cat dorsal spinocerebellar tract neurons after intracellular injection of horseradish peroxidase. J Comp Neurol. 1981 May 20;198(3):453–466. doi: 10.1002/cne.901980306. [DOI] [PubMed] [Google Scholar]
- Randić M., Myslinski N. R., Gordon J. H. Spinal localization of neurons receiving inputs from cutaneous afferents in the cat hindlimb. Brain Res. 1976 Apr 9;105(3):573–577. doi: 10.1016/0006-8993(76)90606-5. [DOI] [PubMed] [Google Scholar]
- Rastad J., Jankowska E., Westman J. Arborization of initial axon collaterals of spinocervical tract cells stained intracellularly with horseradish peroxidase. Brain Res. 1977 Oct 21;135(1):1–10. doi: 10.1016/0006-8993(77)91047-2. [DOI] [PubMed] [Google Scholar]
- Rustioni A., Kaufman A. B. Identification of cells or origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res. 1977 Jan 18;27(1):1–14. doi: 10.1007/BF00234821. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Mann M. D., Brown P. B., Cogdell B. Cells of origin of the cutaneous subdivision of the dorsal spinocerebellar tract. Brain Res. 1975 Feb 21;85(1):59–63. doi: 10.1016/0006-8993(75)91005-7. [DOI] [PubMed] [Google Scholar]


