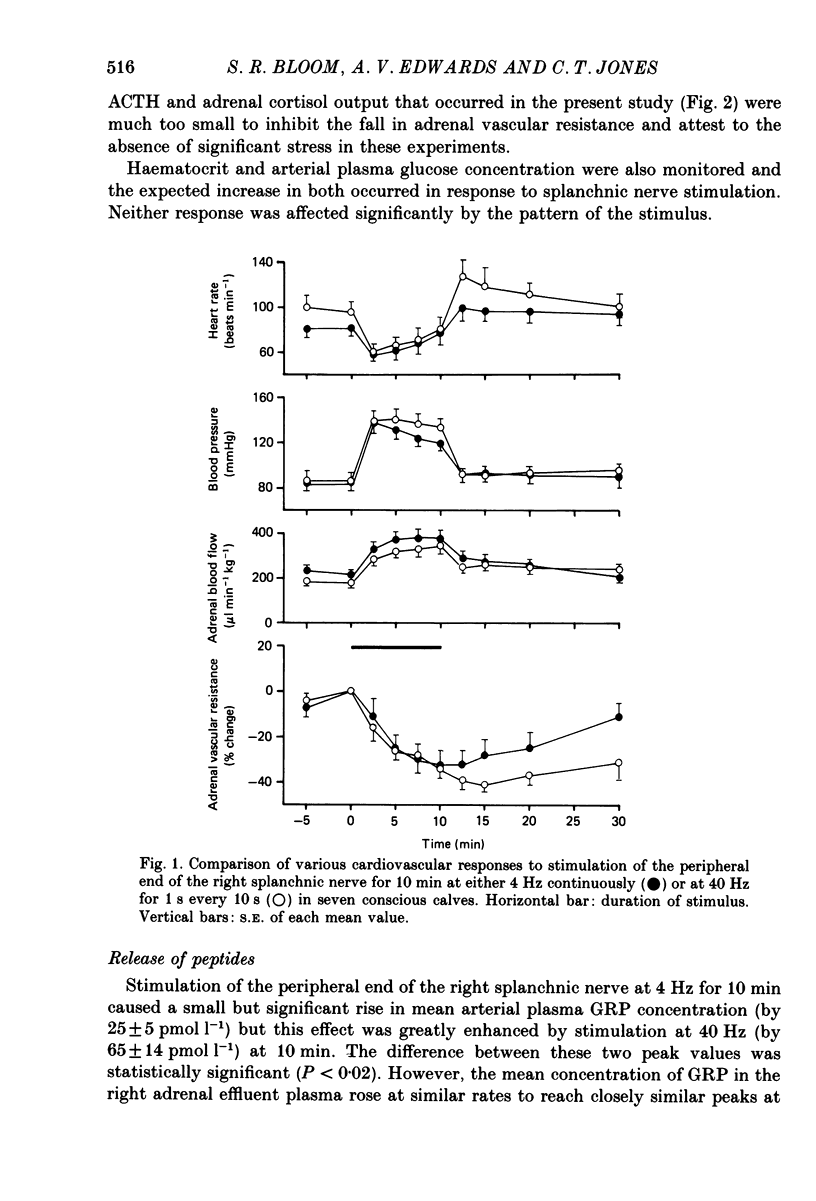
Abstract
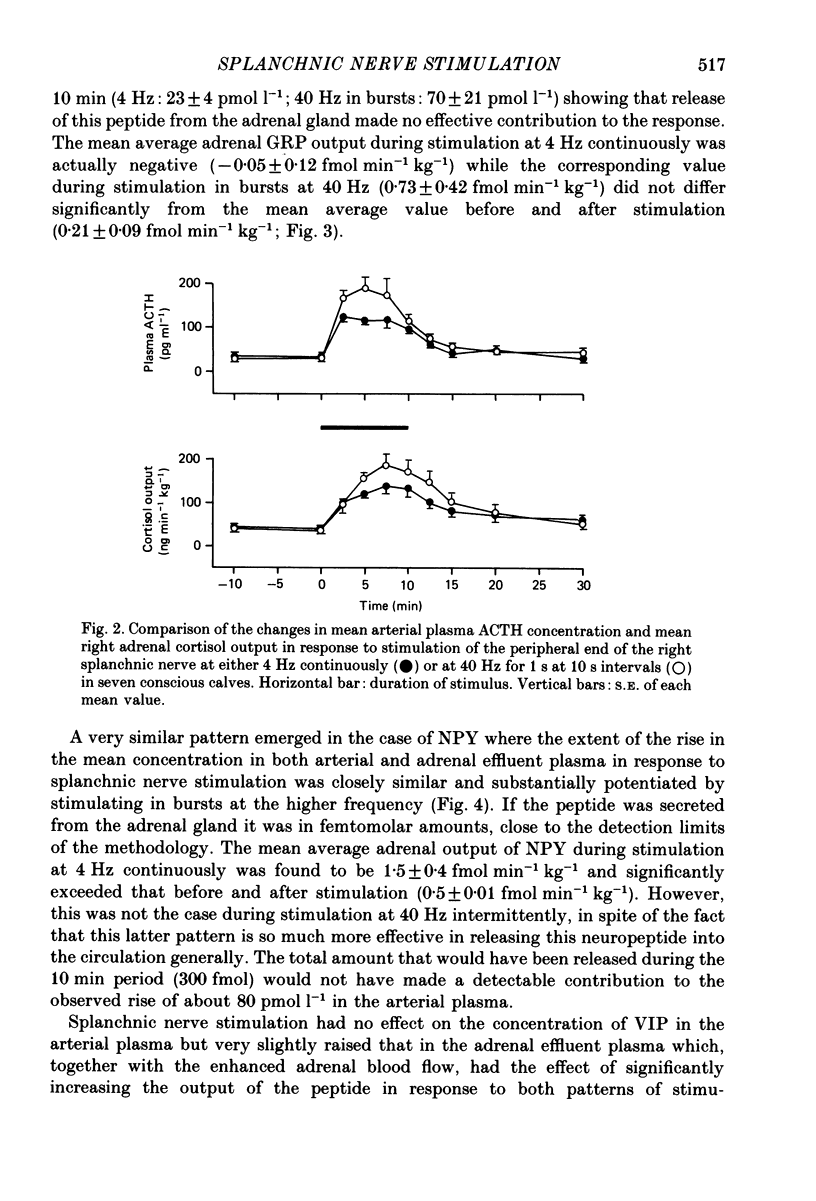
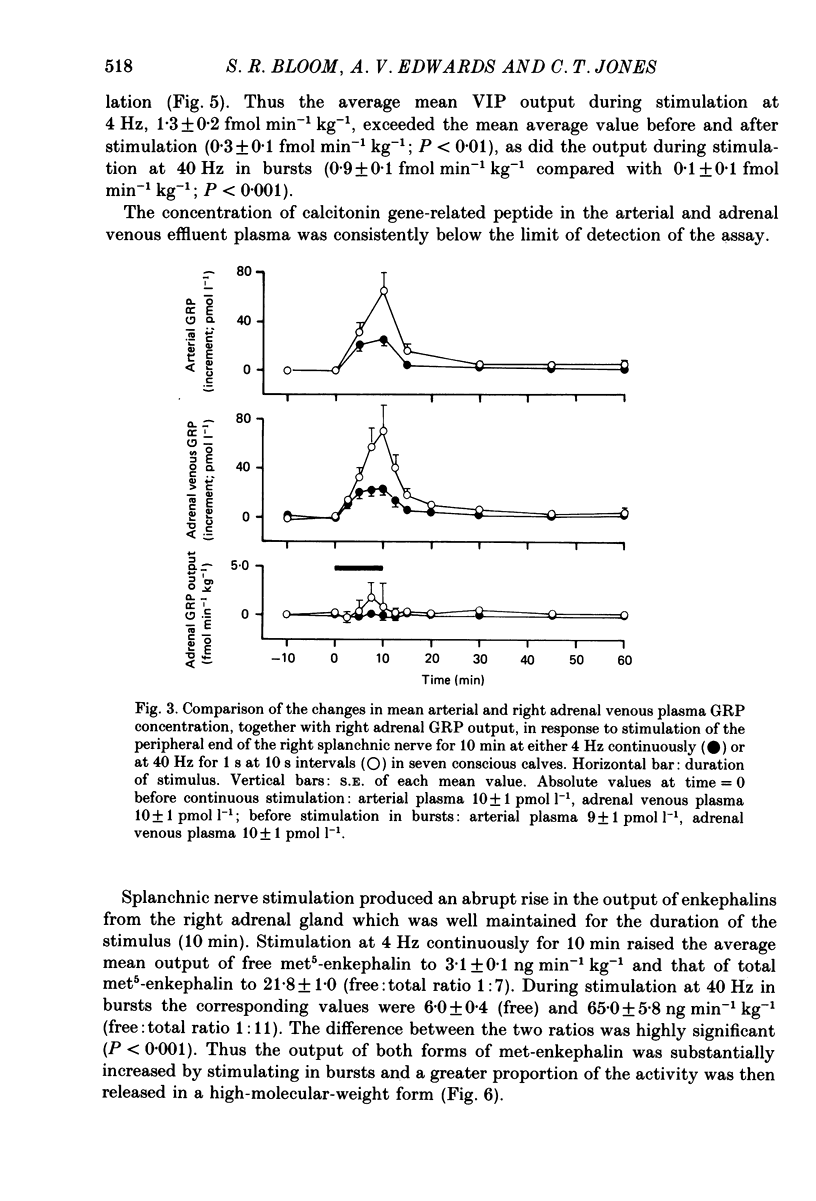
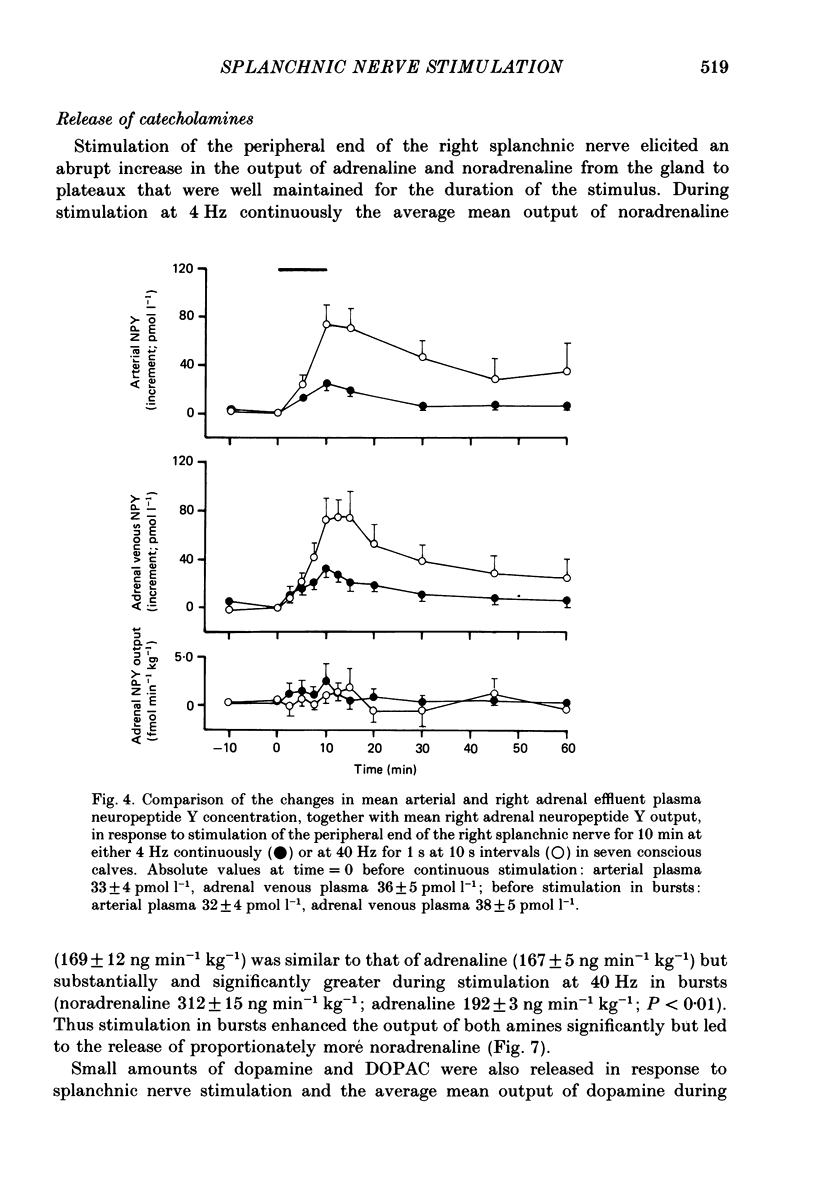
1. The extent to which the adrenal gland contributes to neuroendocrine responses to electrical stimulation of the peripheral end of the splanchnic nerve has been investigated in conscious calves in which the right nerve was stimulated either at 4 Hz continuously for 10 min or at 40 Hz in 1 s bursts at 10 s intervals for the same period. 2. It was confirmed that the release of neuropeptide Y (NPY) and of gastrin-releasing peptide (GRP) is potentiated by stimulation in bursts at a relatively high frequency and shown that the adrenal gland made a negligible contribution to these responses. 3. There was no detectable change in the concentration of vasoactive intestinal peptide (VIP) in the arterial plasma but the existence of a very small but highly significant rise in the output of VIP from the adrenal provided evidence that it was released within the gland in response to splanchnic nerve stimulation. 4. The concentration of calcitonin gene-related peptide (CGRP) in the arterial and adrenal venous effluent plasma was consistently below the level of detection of the assay. 5. Splanchnic nerve stimulation resulted in an abrupt rise in the output of both free and total met5-enkephalin-like immunoreactivity from the adrenal gland which was substantially potentiated by stimulating in bursts. This pattern of stimulation also increased the proportion released in a high-molecular-weight form. 6. Stimulation in bursts significantly enhanced the output of both adrenaline and noradrenaline from the adrenal and resulted in the release of proportionately more noradrenaline. Small amounts of dopamine and DOPAC were also released during splanchnic nerve stimulation and the output of dopamine was significantly increased by stimulating in bursts. 7. Both patterns of stimulation elicited an abrupt rise in mean plasma adrenocorticotrophic hormone (ACTH) concentration, which was associated with an increase in mean adrenal cortisol output and the former effect was significantly enhanced by stimulating in bursts. 8. It is concluded that certain responses to splanchnic nerve stimulation are significantly potentiated by an intermittent high-frequency pattern of stimulation, including all those that are attributable to adrenal medullary activity, whereas others are apparently unaffected by changes in stimulus pattern.
Full text
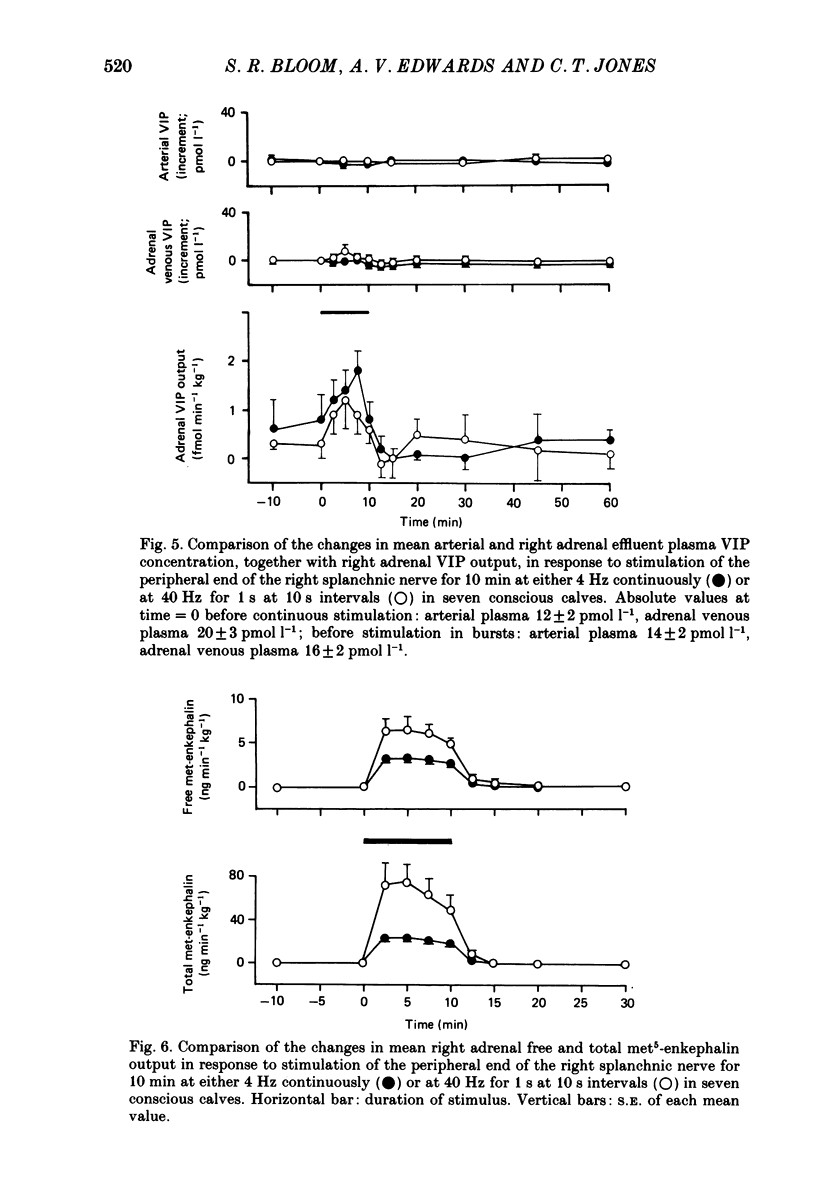
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
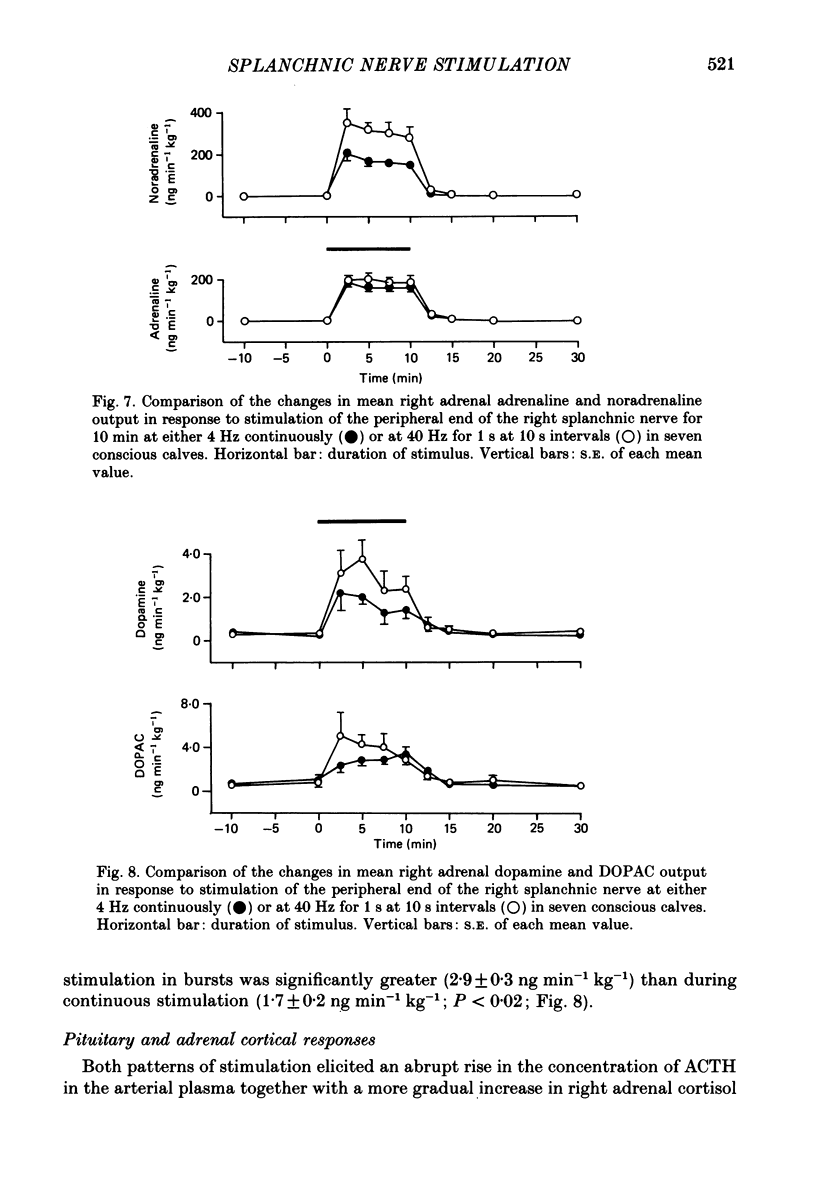
- Ahmed C. E., Dees W. L., Ojeda S. R. The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology. 1986 Apr;118(4):1682–1689. doi: 10.1210/endo-118-4-1682. [DOI] [PubMed] [Google Scholar]
- Allen J. M., Adrian T. E., Polak J. M., Bloom S. R. Neuropeptide Y (NPY) in the adrenal gland. J Auton Nerv Syst. 1983 Nov;9(2-3):559–563. doi: 10.1016/0165-1838(83)90013-9. [DOI] [PubMed] [Google Scholar]
- Allen J. M., Bircham P. M., Bloom S. R., Edwards A. V. Release of neuropeptide Y in response to splanchnic nerve stimulation in the conscious calf. J Physiol. 1984 Dec;357:401–408. doi: 10.1113/jphysiol.1984.sp015507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. M., Yeats J. C., Adrian T. E., Bloom S. R. Radioimmunoassay of neuropeptide Y. Regul Pept. 1984 Jan;8(1):61–70. doi: 10.1016/0167-0115(84)90029-6. [DOI] [PubMed] [Google Scholar]
- Andersson P. O., Bloom S. R., Edwards A. V. Parotid responses to stimulation of the parasympathetic innervation in bursts in weaned lambs. J Physiol. 1982 Sep;330:163–174. doi: 10.1113/jphysiol.1982.sp014335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkinstall S. J., Jones C. T. Regional changes in catecholamine content of the pregnant uterus. J Reprod Fertil. 1985 Mar;73(2):547–557. doi: 10.1530/jrf.0.0730547. [DOI] [PubMed] [Google Scholar]
- Bacopoulos N. G., Hattox S. E., Roth R. H. 3,4-Dihydroxyphenylacetic acid and homovanillic acid in rat plasma: possible indicators of central dopaminergic activity. Eur J Pharmacol. 1979 Jun 15;56(3):225–236. doi: 10.1016/0014-2999(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Garrett J. R. Effects of stimulating the sympathetic innervation in bursts on submandibular vascular and secretory function in cats. J Physiol. 1987 Dec;393:91–106. doi: 10.1113/jphysiol.1987.sp016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Ghatei M. A. Neuroendocrine responses to stimulation of the splanchnic nerves in bursts in the conscious adrenalectomized calf. J Physiol. 1984 Jan;346:519–531. doi: 10.1113/jphysiol.1984.sp015038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Jones C. T. Adrenal cortical responses to vasoactive intestinal peptide in conscious hypophysectomized calves. J Physiol. 1987 Oct;391:441–450. doi: 10.1113/jphysiol.1987.sp016748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V. Vasoactive intestinal peptide in relation to atropine resistant vasodilatation in the submaxillary gland of the cat. J Physiol. 1980 Mar;300:41–53. doi: 10.1113/jphysiol.1980.sp013150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C. Y., Holzwarth M. A. Fetal adrenal VIP: distribution and effect on medullary catecholamine secretion. Peptides. 1986 May-Jun;7(3):413–418. doi: 10.1016/0196-9781(86)90007-0. [DOI] [PubMed] [Google Scholar]
- Davoren J. B., Hsueh A. J. Vasoactive intestinal peptide: a novel stimulator of steroidogenesis by cultured rat granulosa cells. Biol Reprod. 1985 Aug;33(1):37–52. doi: 10.1095/biolreprod33.1.37. [DOI] [PubMed] [Google Scholar]
- Edwards A. V. Adrenal catecholamine output in response to stimulation of the splanchnic nerve in bursts in the conscious calf. J Physiol. 1982 Jun;327:409–419. doi: 10.1113/jphysiol.1982.sp014239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Furness P. N., Helle K. B. Adrenal medullary responses to stimulation of the splanchnic nerve in the conscious calf. J Physiol. 1980 Nov;308:15–27. doi: 10.1113/jphysiol.1980.sp013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hansell D., Jones C. T. Effects of synthetic adrenocorticotrophin on adrenal medullary responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1986 Oct;379:1–16. doi: 10.1113/jphysiol.1986.sp016237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The effects of infusions of synthetic adrenocorticotrophin in the conscious calf. J Physiol. 1974 Jun;239(3):477–498. doi: 10.1113/jphysiol.1974.sp010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Jones C. T. The effect of splanchnic nerve stimulation on adrenocortical activity in conscious calves. J Physiol. 1987 Jan;382:385–396. doi: 10.1113/jphysiol.1987.sp016373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Edvinsson L., Wahlestedt C., Uddman R., Håkanson R., Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984 Apr;8(3):225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Diez-Guerra J., Emson P. C., Winkler H. Bovine chromaffin granules: immunological studies with antisera against neuropeptide Y, [Met]enkephalin and bombesin. Neuroscience. 1986 May;18(1):167–174. doi: 10.1016/0306-4522(86)90185-5. [DOI] [PubMed] [Google Scholar]
- Fredericks C. M., Lundquist L. E., Mathur R. S., Ashton S. H., Landgrebe S. C. Effects of vasoactive intestinal polypeptide upon ovarian steroids, ovum transport and fertility in the rabbit. Biol Reprod. 1983 Jun;28(5):1052–1060. doi: 10.1095/biolreprod28.5.1052. [DOI] [PubMed] [Google Scholar]
- Holzwarth M. A. The distribution of vasoactive intestinal peptide in the rat adrenal cortex and medulla. J Auton Nerv Syst. 1984 Nov;11(3):269–283. doi: 10.1016/0165-1838(84)90041-9. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Schultzberg M., Fahrenkrug J. Immunohistochemical evidence for a local VIP-ergic neuron system in the adrenal gland of the rat. Acta Physiol Scand. 1981 Dec;113(4):575–576. doi: 10.1111/j.1748-1716.1981.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Boddy K., Robinson J. S., Ratcliffe J. G. Developmental changes in the responses of the adrenal glands of foetal sheep to endogenous adrenocorticotrophin, as indicated by hormone responses to hypoxaemia. J Endocrinol. 1977 Mar;72(3):279–292. doi: 10.1677/joe.0.0720279. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Roebuck M. M., Walker D. W., Lagercrantz H., Johnston B. M. Cardiovascular, metabolic and endocrine effects of chemical sympathectomy and of adrenal demedullation in fetal sheep. J Dev Physiol. 1987 Aug;9(4):347–367. [PubMed] [Google Scholar]
- Kowal J., Horst I., Pensky J., Alfonzo M. A comparison of the effects of ACTH, vasoactive intestinal peptide, and cholera toxin on adrenal cAMP and steroid synthesis. Ann N Y Acad Sci. 1977 Oct 28;297:314–328. doi: 10.1111/j.1749-6632.1977.tb41863.x. [DOI] [PubMed] [Google Scholar]
- Kraenzlin M. E., Ch'ng J. L., Mulderry P. K., Ghatei M. A., Bloom S. R. Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul Pept. 1985 Mar;10(2-3):189–197. doi: 10.1016/0167-0115(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Lacković Z., Relja M., Neff N. H. Catabolism of endogenous dopamine in peripheral tissues: is there an independent role for dopamine in peripheral neurotransmission? J Neurochem. 1982 May;38(5):1453–1458. doi: 10.1111/j.1471-4159.1982.tb07925.x. [DOI] [PubMed] [Google Scholar]
- Leboulenger F., Leroux P., Delarue C., Tonon M. C., Charnay Y., Dubois P. M., Coy D. H., Vaudry H. Co-localization of vasoactive intestinal peptide (VIP) and enkephalins in chromaffin cells of the adrenal gland of amphibia. Stimulation of corticosteroid production by VIP. Life Sci. 1983 Jan 24;32(4):375–383. doi: 10.1016/0024-3205(83)90083-8. [DOI] [PubMed] [Google Scholar]
- Linnoila R. I., Diaugustine R. P., Hervonen A., Miller R. J. Distribution of [Met5]- and [Leu5]-enkephalin-, vasoactive intestinal polypeptide- and substance P-like immunoreactivities in human adrenal glands. Neuroscience. 1980;5(12):2247–2259. doi: 10.1016/0306-4522(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Lishajko F. Occurrence and some properties of dopamine containing granules in the sheep adrenal. Acta Physiol Scand. 1968 Jan-Feb;72(1):255–256. doi: 10.1111/j.1748-1716.1968.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Lishajko F. Release, reuptake and net uptake of dopamine, noradrenaline and adrenaline in isolated sheep adrenal medullary granules. Acta Physiol Scand. 1969 May-Jun;76(1):159–171. doi: 10.1111/j.1748-1716.1969.tb04460.x. [DOI] [PubMed] [Google Scholar]
- Mitchell S. J., Bloom S. R. Measurement of fasting and postprandial plasma VIP in man. Gut. 1978 Nov;19(11):1043–1048. doi: 10.1136/gut.19.11.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera A. M., Cathiard A. M., Laburthe M., Saez J. M. Interaction of vasoactive intestinal peptide (VIP) with a mouse adrenal cell line (Y-1): specific binding and biological effects. Biochem Biophys Res Commun. 1979 Sep 12;90(1):78–85. doi: 10.1016/0006-291x(79)91592-4. [DOI] [PubMed] [Google Scholar]
- Morris M. J., Russell A. E., Kapoor V., Cain M. D., Elliott J. M., West M. J., Wing L. M., Chalmers J. P. Increases in plasma neuropeptide Y concentrations during sympathetic activation in man. J Auton Nerv Syst. 1986 Oct;17(2):143–149. doi: 10.1016/0165-1838(86)90089-5. [DOI] [PubMed] [Google Scholar]
- Trzeciak W. H., Ahmed C. E., Simpson E. R., Ojeda S. R. Vasoactive intestinal peptide induces the synthesis of the cholesterol side-chain cleavage enzyme complex in cultured rat ovarian granulosa cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7490–7494. doi: 10.1073/pnas.83.19.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon G. R., Sole M. J. Plasma dopamine: source, regulation, and significance. Metabolism. 1980 Nov;29(11 Suppl 1):1119–1123. doi: 10.1016/0026-0495(80)90020-7. [DOI] [PubMed] [Google Scholar]
- Waldeck B., Snider S. R., Brown R., Carlsson A. Studies on the synthesis and subcellular distribution of dopamine in the rat adrenal medulla. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(1):1–10. doi: 10.1007/BF00632633. [DOI] [PubMed] [Google Scholar]
- de Quidt M. E., Emson P. C. Neuropeptide Y in the adrenal gland: characterization, distribution and drug effects. Neuroscience. 1986 Nov;19(3):1011–1022. doi: 10.1016/0306-4522(86)90313-1. [DOI] [PubMed] [Google Scholar]


