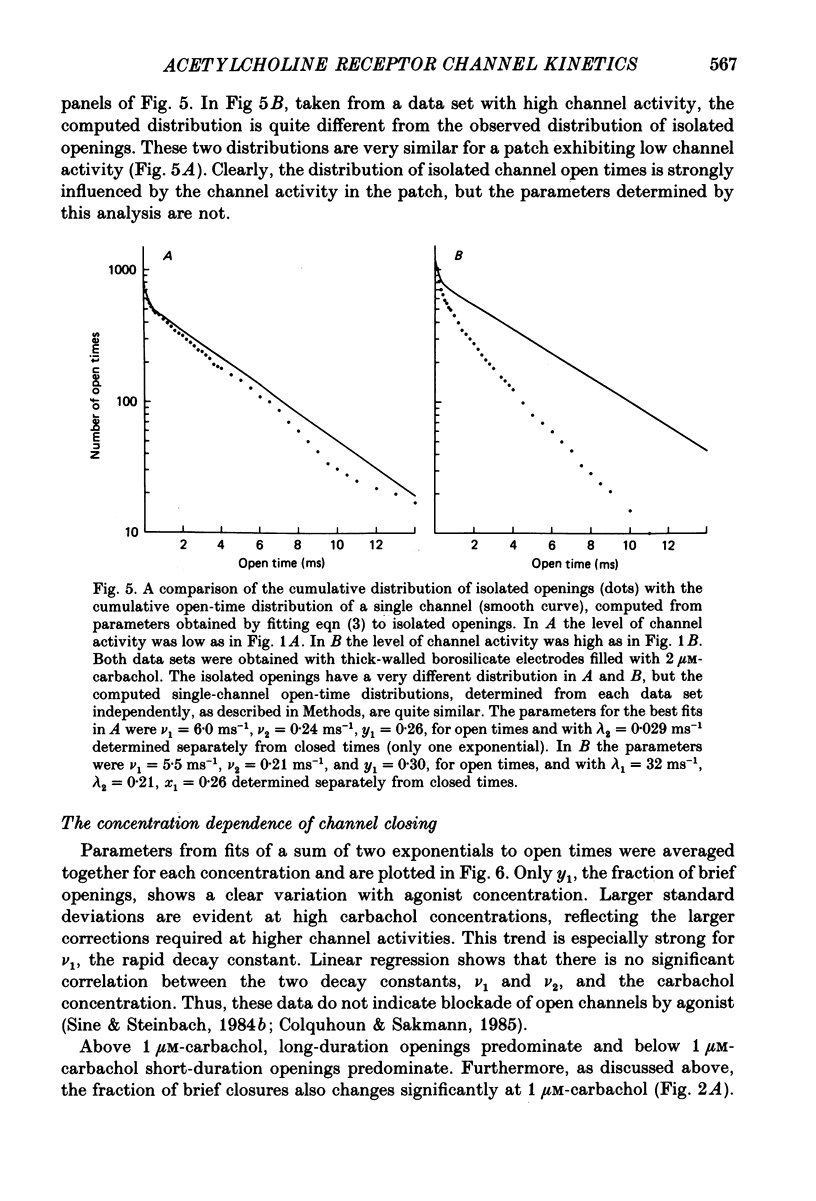
Abstract
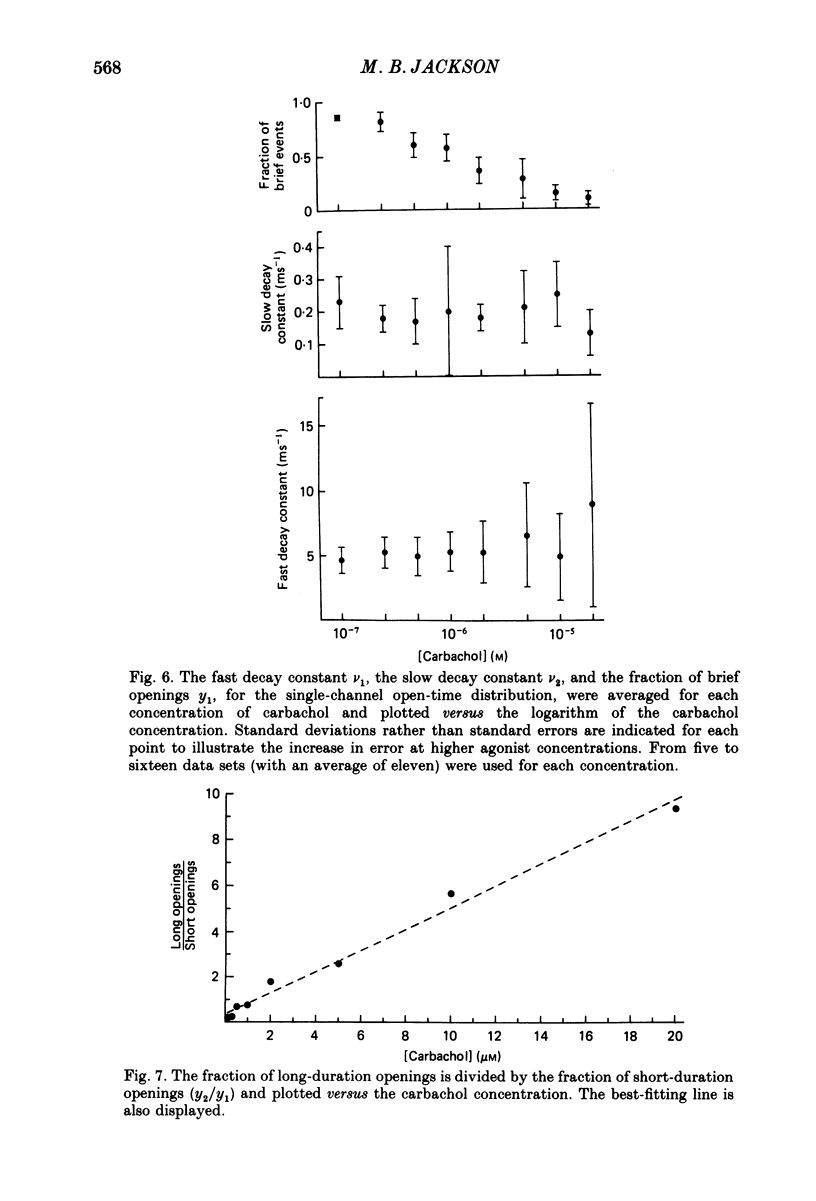
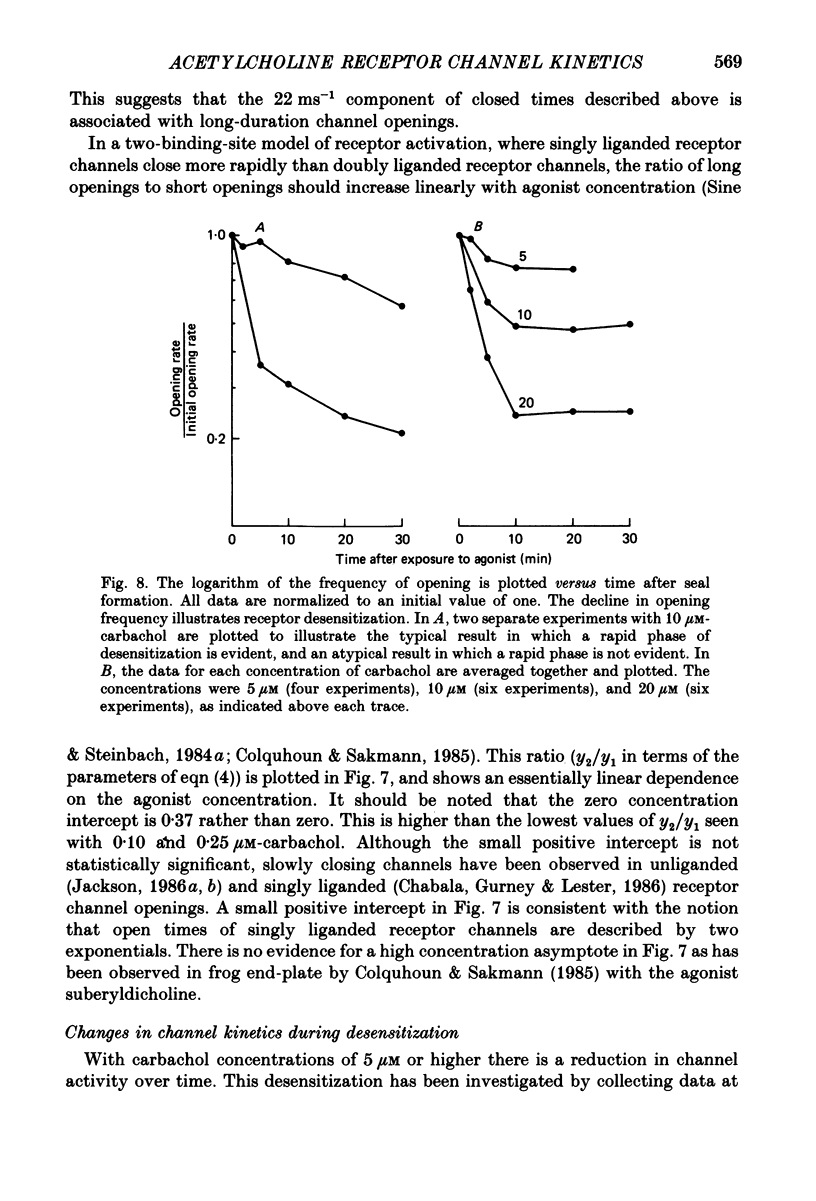
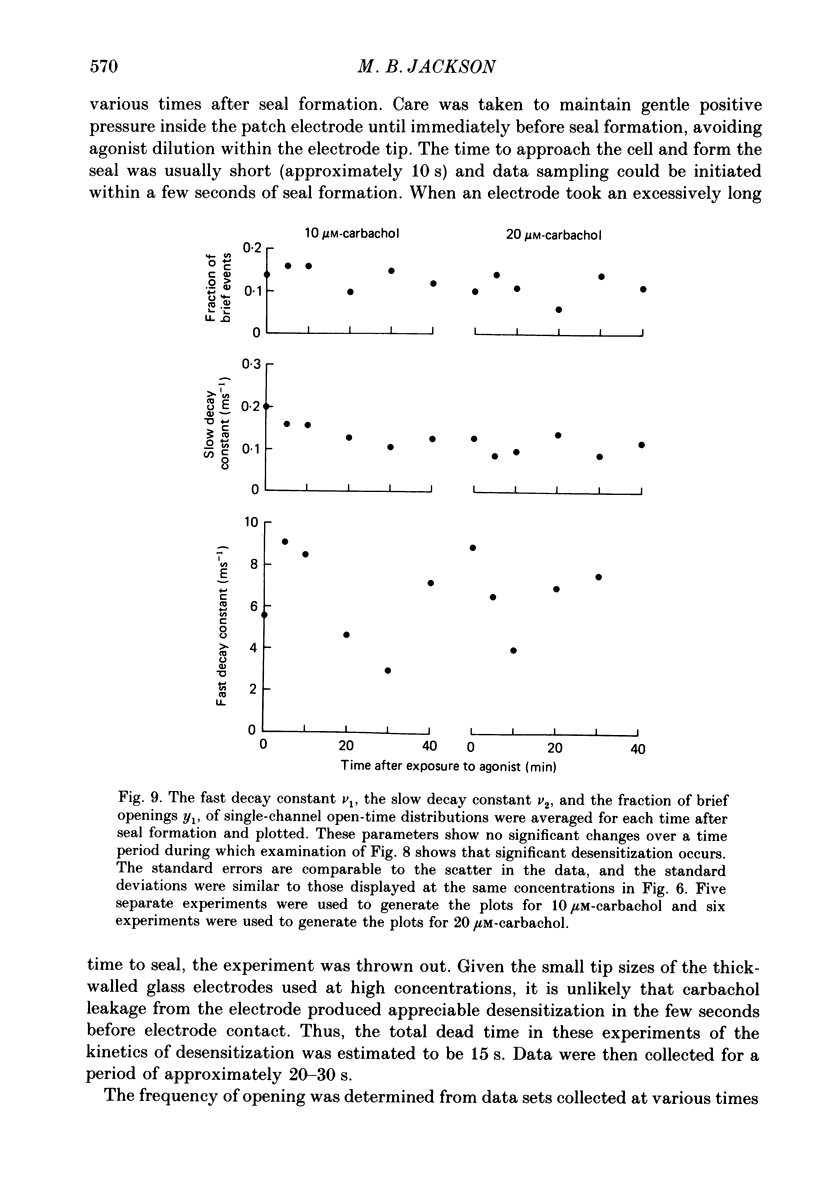
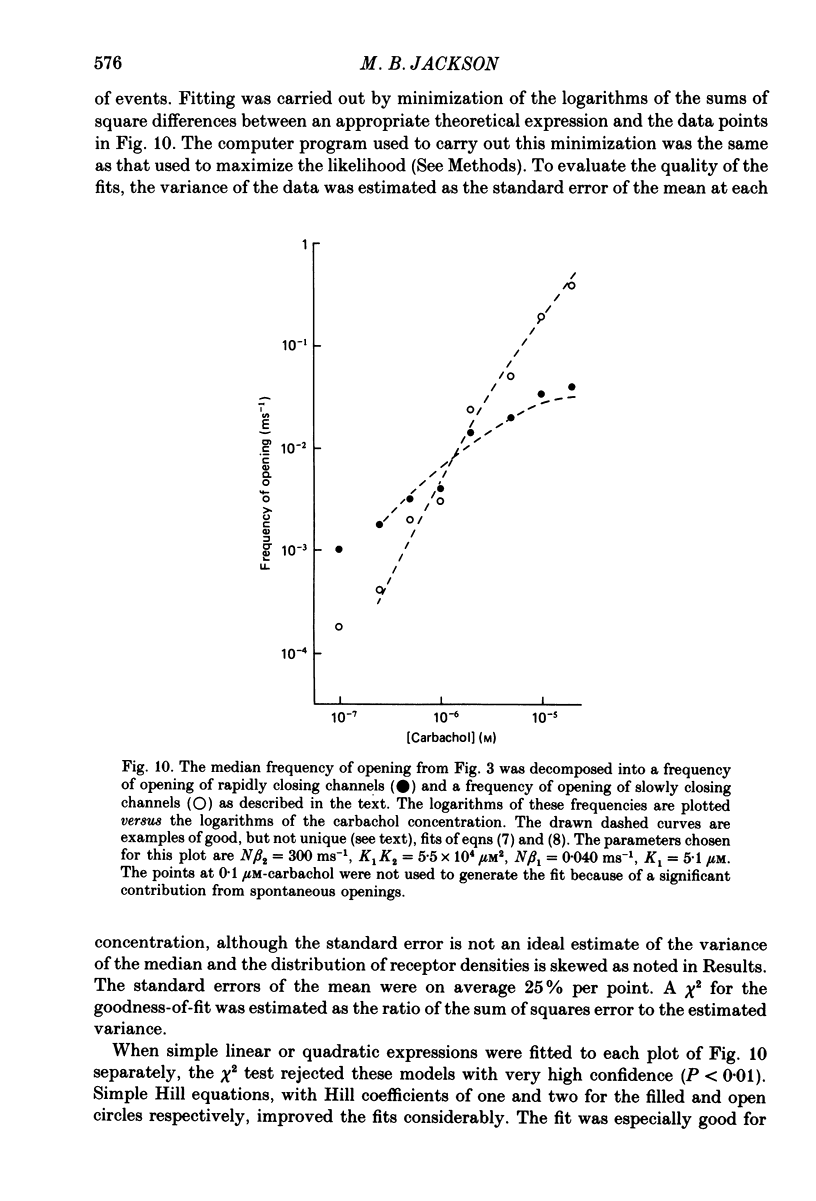
1. The patch-clamp technique was used to study channel gating kinetics of the acetylcholine receptor. The agonist carbachol was used at concentrations varying from 0.10 to 20 microM. 2. Data in which many channels were often open at the same time were analysed with the aid of mathematical expressions that relate the stochastic behaviour of a many-channel system to the kinetic parameters of a single channel. These methods provide consistent estimates of parameters. This consistency suggests that there is no correlation between the kinetics of channel closure and the density of channels in a patch of membrane. 3. Closed times were well fitted by a sum of two exponentials. Addition of a third exponential component never significantly improved the quality of the fit. 4. A sum of two exponentials usually provided the best fit to open times. The ratio of the fractions of slowly closing and rapidly closing channels increased linearly with agonist concentration, in a manner consistent with the opening of singly and doubly liganded receptor channels. 5. Analysis of closed-time densities at various times after seal formation was used to follow the time course of desensitization. No changes in the kinetics of closure were detected during desensitization. 6. At 0.10 microM-carbachol the frequency at which openings were observed was only slightly more than the background frequency of spontaneous opening. At 20 microM-carbachol, immediately after seal formation and before the onset of desensitization, the frequency of opening was approximately 300 times higher. 7. The frequency of appearance of brief-duration openings increased linearly with carbachol concentration and saturated at approximately 5 microM. The frequency of appearance of long-duration openings increased as the square of the agonist concentration, with only a slight hint of saturation. 8. The results presented here are discussed within the framework of a two-binding-site model for the allosteric activation of the acetylcholine receptor. Estimates are made of all of the equilibrium constants and many of the rate constants of the relevant reaction scheme. The two ligand binding sites are found to be very different in terms of their dissociation constants and their influence on the channel gating transitions. These results have implications for the energetics of receptor activation and for the utilization of binding energy by the receptor.
Full text
PDF


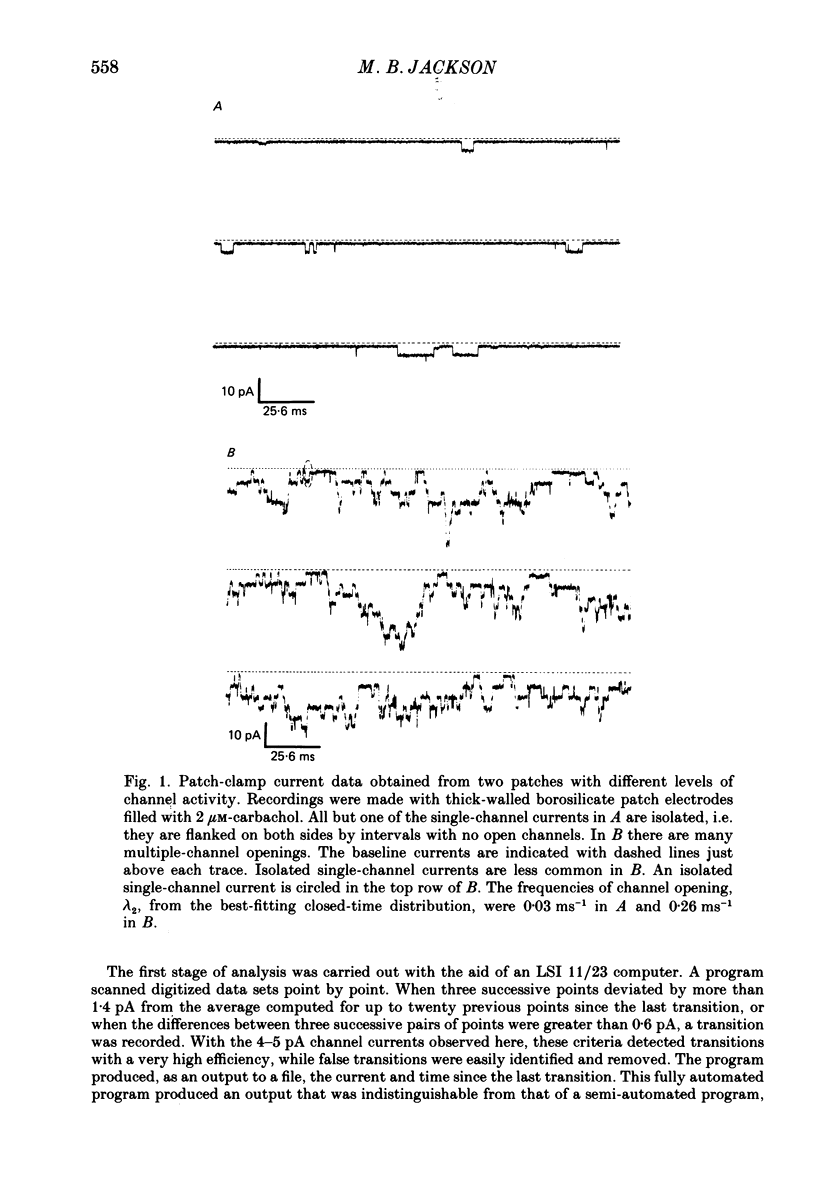


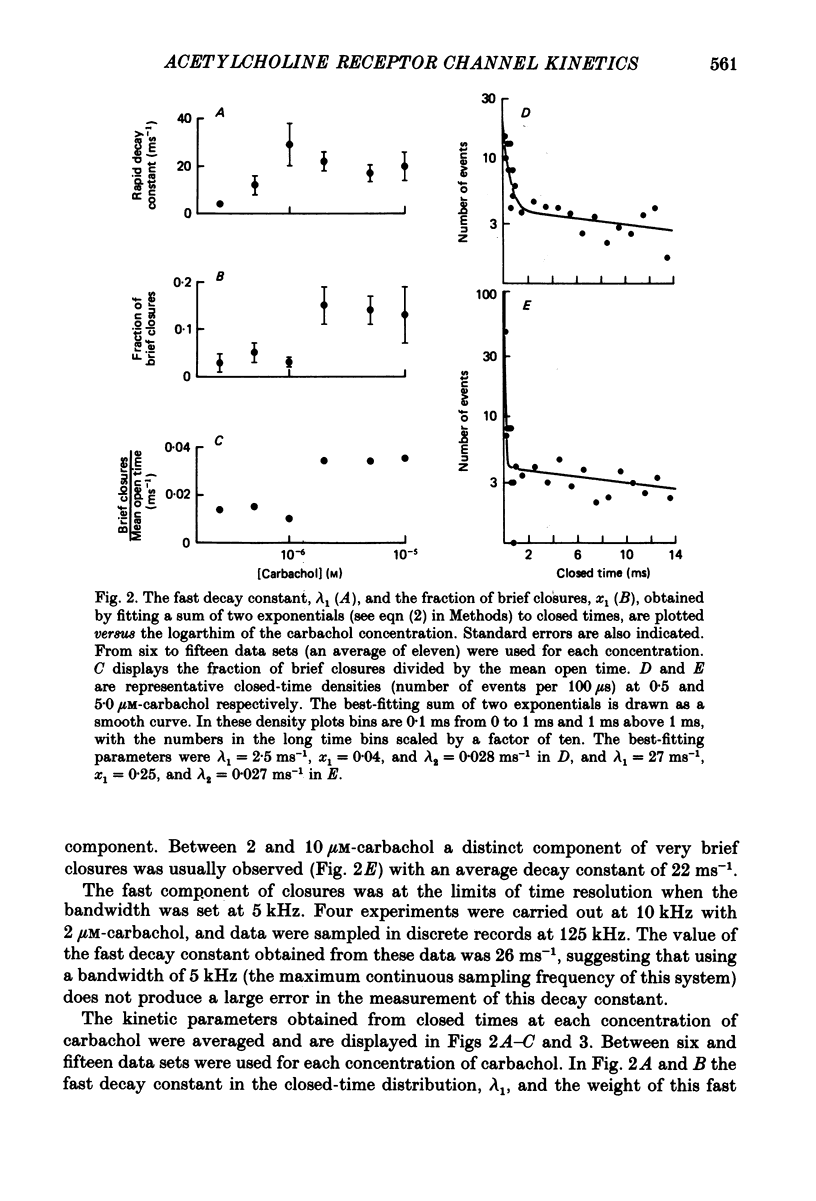

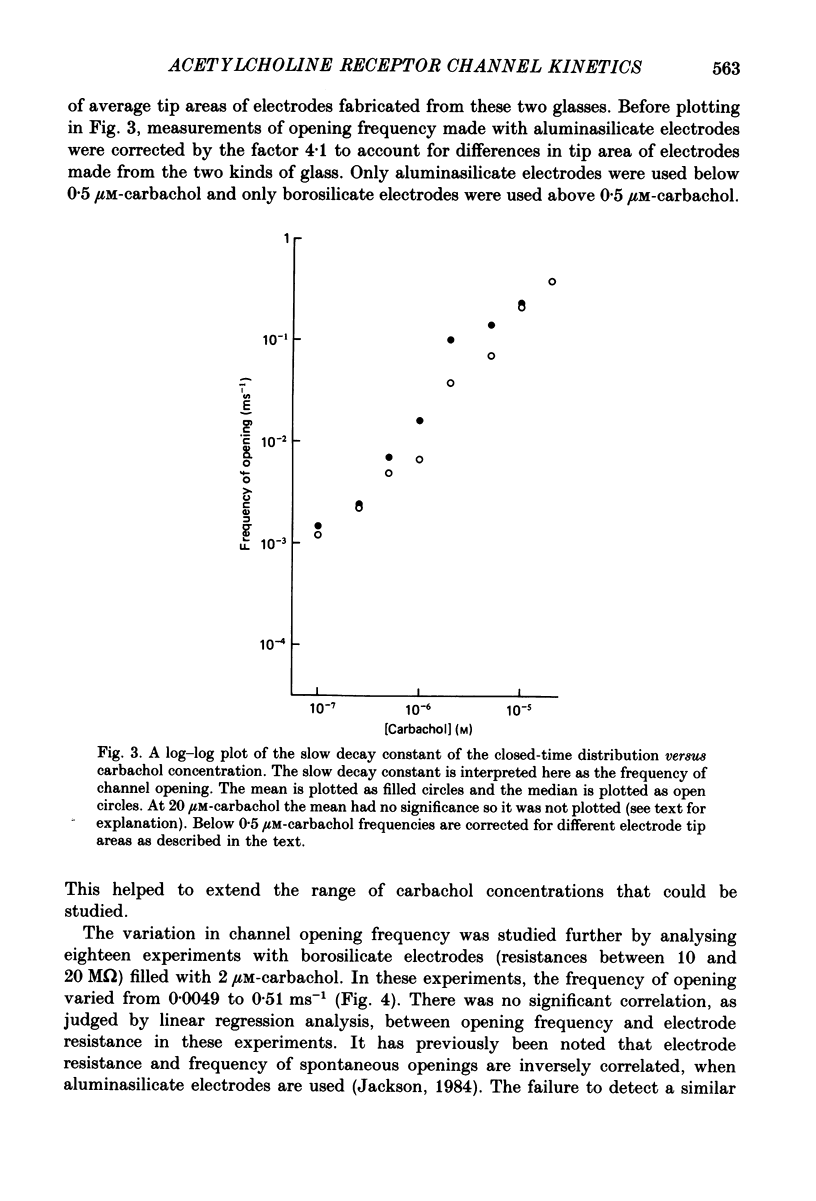


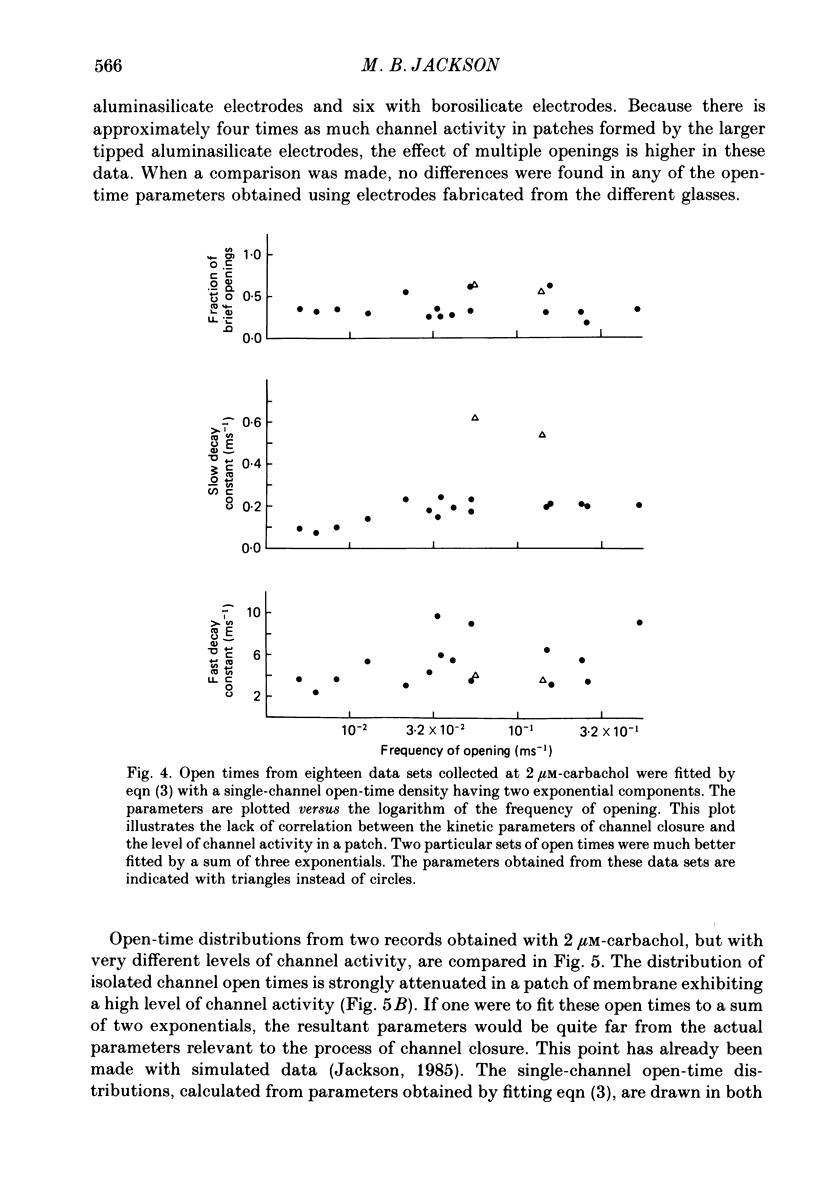












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Auerbach A., Lingle C. J. Activation of the primary kinetic modes of large- and small-conductance cholinergic ion channels in Xenopus myocytes. J Physiol. 1987 Dec;393:437–466. doi: 10.1113/jphysiol.1987.sp016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Lingle C. J. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986 Sep;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Chabala L. D., Gurney A. M., Lester H. A. Dose-response of acetylcholine receptor channels opened by a flash-activated agonist in voltage-clamped rat myoballs. J Physiol. 1986 Feb;371:407–433. doi: 10.1113/jphysiol.1986.sp015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Kinetics of unliganded acetylcholine receptor channel gating. Biophys J. 1986 Mar;49(3):663–672. doi: 10.1016/S0006-3495(86)83693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Spontaneous openings of the acetylcholine receptor channel. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3901–3904. doi: 10.1073/pnas.81.12.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Stochastic behavior of a many-channel membrane system. Biophys J. 1985 Feb;47(2 Pt 1):129–137. doi: 10.1016/s0006-3495(85)83886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Labarca P., Montal M. S., Lindstrom J. M., Montal M. The occurrence of long openings in the purified cholinergic receptor channel increases with acetylcholine concentration. J Neurosci. 1985 Dec;5(12):3409–3413. doi: 10.1523/JNEUROSCI.05-12-03409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Podleski T. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor distribution on myotubes in culture correlated to acetylcholine sensitivity. J Physiol. 1977 Jul;269(1):155–176. doi: 10.1113/jphysiol.1977.sp011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Middleton P., Jaramillo F., Schuetze S. M. Forskolin increases the rate of acetylcholine receptor desensitization at rat soleus endplates. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4967–4971. doi: 10.1073/pnas.83.13.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E., Wong B. S., Jackson M. B., Lecar H. Single-channel currents activated by curare in cultured embryonic rat muscle. J Neurosci. 1983 Dec;3(12):2525–2531. doi: 10.1523/JNEUROSCI.03-12-02525.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M., Frank E. F., Fischbach G. D. Channel open time and metabolic stability of synaptic and extrasynaptic acetylcholine receptors on cultured chick myotubes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):520–523. doi: 10.1073/pnas.75.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono S., Takeyasu K., Udgaonkar J. B., Delcour A. H., Fujita N., Hess G. P. Regulatory properties of acetylcholine receptor: evidence for two different inhibitory sites, one for acetylcholine and the other for a noncompetitive inhibitor of receptor function (procaine). Biochemistry. 1984 Dec 18;23(26):6889–6893. doi: 10.1021/bi00321a094. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Taylor P. Relationship between reversible antagonist occupancy and the functional capacity of the acetylcholine receptor. J Biol Chem. 1981 Jul 10;256(13):6692–6699. [PubMed] [Google Scholar]
- Takeda K., Trautmann A. A patch-clamp study of the partial agonist actions of tubocurarine on rat myotubes. J Physiol. 1984 Apr;349:353–374. doi: 10.1113/jphysiol.1984.sp015160. [DOI] [PMC free article] [PubMed] [Google Scholar]


