Abstract
The primary goal of antiretroviral therapy (ART) is to suppress viral replication to undetectable levels (< 50 copies/mL). Despite achieving complete viral suppression, 10–40% of individuals on ART do not adequately restore their CD4 + T-cell count, being defined as immunological non-responders (INR). Factors such as sex, age at treatment initiation, coinfections, and pre-ART CD4 + T-cell count may influence this insufficient recovery. This impairment can also result from poor production or exacerbated destruction of CD4 + T-cells, particularly through extrinsic pathway-mediated apoptosis involving Fas/FasL and caspase-3. Thus, this study aimed to evaluate the expression profile of extrinsic apoptosis pathway genes (CASP3, FAS, FASLG) in adult male HIV patients on ART. The patients were stratified as immunological responders (n = 25) and immunological non-responders (n = 8) based on the increase and total count of CD4 + T-cells. Significant differences for CASP3 (FC = 1.39, p = 0.047) and FASLG (FC = 1.94, p < 0.0001) gene expressions were identified between IR and INR groups, but not for FAS (FC=-1.2, p = 0.638). This study indicates increased apoptotic pathway gene expression in INR and highlights the influence of cell destruction mechanisms on immunological recovery.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-10665-4.
Keywords: CD4 + T-cell recovery, immunological non-responders, cell death, CASP3, FAS, FASL
Introduction
The human immunodeficiency virus (HIV) is a single-stranded retrovirus that infects and destroys immune cells, particularly CD4 + T-cells, which play a crucial role in coordinating the immune response [1]. People living with HIV (PLHIV) experience a chronic depletion of these cells, which can lead to the progression to acquired immunodeficiency syndrome (AIDS) is established [2]. AIDS is characterized by severe immune system compromise, increasing susceptibility to opportunistic infections and HIV-related cancers [3, 4]. Antiretroviral therapy (ART) seeks to achieve virological suppression, defined as reducing the viral load to undetectable levels (< 50 copies/mL) [5, 6].
Although not the primary goal, a gradual reconstitution of CD4 + T-cells is expected in PLHIV undergoing successful ART [7]. Patients who achieve a satisfactory immune reconstitution, indicated by an increase in CD4 + T-cell count, are classified as immunological responders (IR) [8]. However, 10–40% of individuals who achieve virological suppression exhibit incomplete immune reconstitution, defined as immunological non-responders (INR). These individuals experience limited CD4 + T-cell recovery despite prolonged ART, typically gaining less than 200 cells/µL over 24 months and failing to reach a total CD4 + count of 500 cells/µL. As a result, INR are more susceptible to developing HIV-related complications [9].
Incomplete immune reconstitution is a multifactorial condition influenced by several factors such as advanced age, male sex, exacerbated immune activation, thymic exhaustion, co-infections, genetic background, and low pre-ART CD4 + T-cell counts. Immunological recovery depends on a delicate balance between CD4 + T-cell production and destruction [7, 9, 10]. Studies demonstrate that immunological nonresponse is primarily characterized by reduced T-cell production and increased rates of T-cell destruction [11, 12]. In the context of HIV infection, apoptosis plays a key role in CD4 + T-cell depletion, as viral proteins possess apoptotic properties that induce the death of both infected and, notably, uninfected cells [13, 14].
Apoptosis is a programmed cell death process where cells undergo self-destruction in a regulated manner, occurring via two primary pathways: intrinsic and extrinsic [15–17]. The extrinsic apoptosis pathway is initiated by the activation of death receptors on immune cells, such as the Fas receptor (CD95) [17]. The Fas ligand (FasL or CD178), expressed on immune cells like lymphocytes and natural killer cells [18], binds to FAS, triggering a cascade that leads to activation of caspase-3, the key effector of apoptosis [19]. The Fas-FasL pathway is a major mechanism driving apoptosis in immune cells, and dysregulation of this pathway can contribute to the development of immunological nonresponse in PLHIV undergoing ART [20, 21].
Moreover, as previously mentioned, sex can influence immune reconstitution, with male ART-treated PLHIV being more prone to immunological nonresponse [12, 22]. Thus, this study aimed to evaluate the association between the expression of genes encoding proteins involved in the extrinsic apoptosis pathway (FAS, FASLG, and CASP3) in male ART-treated patients, and to explore a potential relation between these gene expressions and immune reconstitution.
Materials and methods
Study population
This study included 33 male ART-treated PLHIV, recruited from the Instituto de Medicina Integral Professor Fernando Figueira (IMIP) in Pernambuco, Northeast Brazil, between 2018 and 2020. The inclusion criteria were being male, ≥ 18 years old, on ART for at least 24 months with good adherence, and exhibiting undetectable viral load. The exclusion criteria included cancer, autoimmune diseases, and history of injecting drug use. Clinical data were obtained from medical records. This study was approved by the Ethics Committee of IMIP (protocol code: 3629-13).
Immunological classification
The patients were classified as IR and INR based on their CD4 + T-cell count after 24 months of ART. Patients who either started ART with a baseline CD4 + count of ≥ 500 cells/µL or achieved this count after 24 months were classified as IR. Conversely, patients who began ART with < 500 cells/µL and gained < 200 cells/µL during treatment were classified as INR. As a result, seven male ART-treated PLHIV were classified as INR, and 25 patients were defined as IR.
PBMC isolation
Peripheral blood samples (4mL) were collected in EDTA tubes. Peripheral blood mononuclear cells (PBMC) were then isolated using Ficoll-Paque Plus density gradient centrifugation, followed by two washes and resuspension in 1x PBS.
Expression analysis
RNA from the 33 ART-treated PLHIV was isolated from the PBMCs suspension using Trizol® (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The RNA concentration and quality were assessed using a Nanodrop® 2000 spectrophotometer (Thermo Fisher Scientific, USA). cDNA was then synthesized employing 500 ng of RNA per sample as input, using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, USA), following the manufacturer’s instructions. The reverse transcription reaction was performed with an initial incubation at 25 °C for 10 min, followed by 120 min at 37 °C, and a final step at 85 °C for 5 min. Gene expression assays for CASP3 (Hs00234387), FAS (Hs00236330), and FASLG (Hs00181226) were performed using Taqman® probes, on an ABI 7500 Real Time PCR detection system (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. The reference genes GAPDH, ACTB, and RPLP0 were used for normalization, and relative gene expression was calculated using the 2−ΔΔCq method.
Statistical analysis
Fisher’s exact and Chi-squared tests were used to evaluate categorical (clinical) variables. The normal distribution of numerical variables, including gene expression data, was assessed using the Shapiro-Wilk test. Variables with a normal distribution were compared between groups using Student’s t-test and are presented as mean ± SD. For variables that did not follow a normal distribution, the Mann-Whitney test was applied, and the results was represented by the median and interquartile range (IQR). Linear regression analysis was performed to correct possible confounding factors in relation to gene expression. The statistical significance level (α) was set at 0.05 for all tests. Statistical analyses were performed using GraphPad Prism, version 8.0.1.
Results
Clinical data analyses
We observed a significant difference in pre-treatment CD4 + T-cell counts between the groups (p = 0.008), with means of 215.5 ± 50.2 cells/µL for the INR group and 473.2 ± 49.0 for the IR group, demonstrating that IRs initiated ART with higher CD4 + T-cell levels. A significant difference was also observed in pre-treatment CD4% (p = 0.004), and this pattern was maintained after 24 months after ART, where both the CD4 + T-cell count (p = 0.004) and the CD4% (p = 0.002) remained significantly different. Additionally, the CD4/CD8 ratio after 24 months of ART showed a notable difference between the two groups (p = 0.011).
In addition, we analyzed age at ART initiation, body mass, time to ART initiation post-diagnosis, ART regimens, and their changes, pre-treatment CD8 + T-cell count, CD8% after 24 months of ART, and pre-treatment plasma viral load (PVL). However, no statistically significant differences were identified between the groups for any of these variables (Table 1).
Table 1.
Clinical characteristics of PLHIV based on their immunological response during ART
| Variables | INR n = 8(%) |
IR n = 25(%) |
p | |
|---|---|---|---|---|
| Age (years old) at ART start date* | 40.7 ± 3.8 | 32.2 ± 2.2 | 0.063 | |
| Body mass (kg)* | 77.6 ± 6.0 | 72.2 ± 2.4 | 0.666 | |
|
Time (weeks) to ART starting post-diagnosis** |
6.0 (2.0-10.7) | 7.0 (2.0–9.0) | 0.756 | |
|
Detailed ART regimens, NRTI + 3TC + (third option) |
ABC + 3TC + DTG | 0 (0.0) | 1 (4.0) | 0.850b |
| TDF + 3TC + ATV/r | 1 (12.5) | 2 (8.0) | ||
| TDF + 3TC + DTG | 5 (62.5) | 13 (52.0) | ||
| TDF + 3TC + EFZ | 2 (25.0) | 9 (36.0) | ||
|
ART regimens, stratified by classes |
2 NRTI + INI | 5 (62.5) | 14 (56.0) | 0.818b |
| 2 NRTI + IP/r | 1 (12.5) | 2 (8.0) | ||
| 2 NRTI + NNRTI | 2 (25.0) | 9 (36.0) | ||
| ART regimen changec | 2 (28.6) | 3 (12.0) | 1.000a | |
|
Pre-treatment CD4 + T-cell count (cells/µL)* |
215.5 ± 50.2 | 473.2 ± 49.0 | 0.008 | |
|
Pre-treatment CD8 + T-cell count (cells/µL)* |
830.4 ± 133.3 | 971.1 ± 85.0 | 0.721 | |
|
CD4 + T-cell count after 24 months of ART (cells/µL)* |
312.0 ± 68.4 | 761.2 ± 77.9 | 0.004 | |
|
CD4/CD8 ratio after 24 months of ART* |
0.4 ± 0.10 | 1.1 ± 0.12 | 0.011 | |
| Pre-CD4+ (%)* | 7.4 ± 3.6 | 21.6 ± 1.8 | 0.004 | |
|
CD4+ (%) after 24 months of ART* |
18.5 ± 2.5 | 34.8 ± 1.9 | 0.0002 | |
|
CD8+ (%) after 24 months of ART* |
44.7 ± 3.6 | 39.0 ± 2.3 | 0.231 | |
|
Pre-treatment PVL (log10 RNA copies/mL)* |
4.1 ± 0.5 | 4.5 ± 0.2 | 0.391 |
* t-test (Shapiro-Wilk test: >0.05), values displayed as mean ± SE
**Wilcoxon-Mann-Whitney test (Shapiro-Wilk: <0.05), values displayed as median (IQR)
aFisher’s exact test
bChi-squared test
cChange from a NNTRI-containing cART (EFZ) to a PI/r (ATV/r), INI (DTG), or another NNTRI (NVP) -containing ART regimen
Expression assays
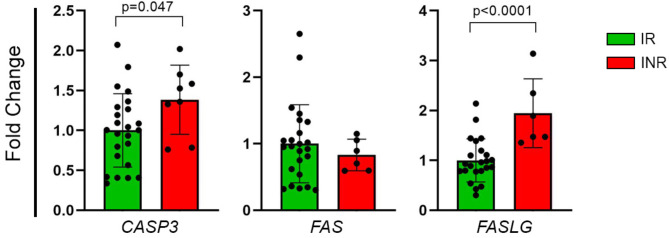
In our study, we evaluated gene expression profiles in 33 men living with HIV undergoing ART, consisting of 25 IR and 8 INR. The results revealed a statistically significant difference in the expression of apoptosis-related genes between the IR and INR groups. Specifically, CASP3 expression was significantly elevated in the INR group, showing a 1.39-fold change (FC) (p = 0.047) (Fig. 1A). Similarly, the FASLG expression was 1.94-FC higher in the INR group, with statistical significance (p < 0.0001) (Fig. 1C). However, despite a slight decrease of 1.2-FC in FAS gene expression, this difference was not statistically significant between the groups (p = 0.638) (Fig. 1B).
Fig. 1.
Differential gene expression of the extrinsic apoptosis pathway between IR and INR: (A)CASP3 (FC = 1.39; p = 0.047); (B)FAS (FC = -1.2; p = 0.638); (C)FASLG (FC = 1.94; p < 0.0001). The relative expression levels of these genes are depicted in scatter plots based on fold change values. The relative expression levels of these genes were analyzed using the 2−ΔΔCq method and compared between groups with the Mann-Whitney test. The data are presented as the mean with the corresponding standard deviation
Although age did not show a statistically significant difference between the groups (p = 0.063), there was a notable difference between them (INR = 40 years old and IR = 32 years old, Table 1). In fact, age is a factor that may influence immune reconstitution and gene expression. To account for this confounding factor, we performed linear regression analyses between age at ART initiation and the gene expression levels of CASP3, FAS and FASL. According to the linear regression models for each gene, age at ART initiation did not significantly influence gene expression in ART-treated PLHIV. The linear regression models are provided in Supplementary Data.
Discussion
Although the primary goal of ART is controlling viral replication, its objectives have expanded to facilitate the recovery of CD4 + T-cells lost during HIV infection [7]. Despite achieving complete virological suppression, a significant proportion of PLHIV continue to have low CD4 + T-cell counts, making them susceptible to opportunistic infections and neoplasms, which adversely affect their quality of life [11].Various mechanisms that influence CD4 + T-cell restoration have been identified, including an exacerbated cell death through apoptosis [23]. Additionally, male sex has also been recognized as a risk factor for immunological nonresponse [12]. Thus, the present study aimed to evaluate clinical factors associated with immune reconstitution, and the expression levels of apoptosis-related genes in male ART-treated PLHIV.
One of the most significant factors influencing immune reconstitution in PLHIV is the pre-ART CD4 + T-cell count [24]. Our analyses demonstrate that the IR group shows a higher pre-ART CD4 + T-cell count, aligning with other studies that have associated low levels of CD4 + T-cells at the beginning of ART with immunological nonresponse [25–27]. Patients who initiate ART with a baseline CD4 + T-cell ≥ 500 cells/µL have a reduced risk of mortality and progression to AIDS, as well as a higher change of satisfactory immunological recovery and maintaining an undetectable viral load [27]. This underscores the importance of early diagnosis and supports the “treat-all” approach recommended by the World Health Organization (WHO) [28].
Our study also reveals that the INR group exhibits significantly higher expression of genes involved in extrinsic apoptosis pathways, such as CASP3 and FASLG. Previous studies have identified elevated surface expression of apoptotic markers, such as annexin-V, and increased levels of pro-apoptotic proteins in INR compared to IR, suggesting that apoptosis plays a crucial role in CD4 + T-cell destruction [29, 30]. While apoptosis is a physiological process essential for maintaining T-cell homeostasis, its excessive activation can critically impact immunological recovery of PLHIV during ART [7].
Several mechanisms may contribute to the increased expression of apoptosis-related genes, including exacerbated immune activation [31, 32]. It has been established that a high levels of activated CD4 + T-cells undergo apoptosis [33]. Our previous research also demonstrated that INR group exhibits higher immune activation, especially within CD4 + T-cells [25]. Additionally, studies have shown that, in conjunction with exacerbated immune activation, CD4 + T-cells experience increased cellular proliferation rates and a higher incidence of spontaneous apoptosis, effectively mediated by caspase-3 activity [23, 32, 34]. Different apoptosis pathways contribute to CD4 + T-cell death during HIV infection, including both intrinsic (mitochondria-mediated) and extrinsic (death receptor-mediated) pathways. The extrinsic pathway, in particular, can be triggered by TNF-family ligands, such as FasL, leading to caspase-3 activation and apoptosis [16, 17, 35]. Moreover, pyroptosis is another mechanism of cell death that has been implicated in CD4 + T-cell depletion during HIV infection. Pyroptosis, driven by inflammasome activation, could contribute to the loss of CD4 + T-cells in INR [2, 10, 15].
Another mechanism associated with immune activation and closely linked to apoptosis is T-cell exhaustion [36]. There is a positive correlation between increased levels of apoptosis and the expression of PD-1, a key marker of T-cell exhaustion during HIV infection. This suggests that exhausted CD4 + T-cells in the INR group are particularly prone to apoptosis [37]. These findings indicate the intrinsic connection between immune activation and apoptosis, which significantly contributes to immunological nonresponse in PLHIV undergoing ART [33].
Although our study did not identify a direct association between FAS expression and incomplete immune reconstitution, the FAS receptor plays an important role in the extrinsic apoptosis pathway [19]. Post-transcriptional modifications or other genetic variations in the FAS receptor could potentially contribute to immunological nonresponse [38].
The present study has some limitations. The small sample size, particularly in the INR group (n = 8), may impact on the statistical power and limit the generalizability of our findings. Additionally, gene expression analysis was performed using whole PBMCs rather than isolated CD4 + T-cells, which may obscure cell-specific gene expression patters. Future studies involving larger cohorts and cell-type-specific analyses are warranted to validate these results.
Conclusion
Our data suggest that the increased expression of genes related to the extrinsic apoptosis pathway, such as FASLG and CASP3, plays a crucial role in the immunological nonresponse of men living with HIV undergoing ART. This demonstrates that the molecular and genetic mechanisms involved in CD4 + T-cell depletion may impact the immune reconstitution of ART-treated PLHIV. Given the multifactorial nature of immunological nonresponse, further studies should include additional genes involved in alternative extrinsic and intrinsic apoptosis pathways, as well as other cell death mechanisms. Moreover, clinical factors should be evaluated across diverse populations, as our study highlighted that individual clinical variable can significantly influence immune outcome to ART.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
H.F.L.-A. and M.C.S.G. were responsible for the study design, gene expression and clinical analyses, and writing of the manuscript. W.H.V.C.-S; L.C.A.A.; L.M.L.M. and R.L.G. were responsible for the study design. All authors were involved in manuscript review and editing. H.F.L.-A. and M.C.S.G. wrote the authorship section of this work and should be considered as co-first authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by CNPq (403462/2023-1 to R.L.G.), FACEPE (APQ-0599-2.02/14 to R.L.G.) and UFPE (PROPG and PROPESQI to R.L.G.) grants.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Instituto de Medicina Integral Professor Fernando Figueira (protocol code: 3629-13; 13 November 2013). Informed consent was obtained from all subjects involved in this study.
Consent for publication
Not applicable.
Clinical trial
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Henrique Fernando Lopes-Araujo and Maria Carolina Santos Guedes contributed equally to this work.
References
- 1.Balasubramaniam M, Pandhare J, Dash C. Immune control of HIV. J Life Sci. 2019;1:4–37. [PMC free article] [PubMed] [Google Scholar]
- 2.Doitsh G, Greene WC. Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe. 2016;19:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiao EY, Coghill A, Kizub D, Fink V, Ndlovu N, Mazul A, Sigel K. The effect of Non-AIDS-Defining cancers on people living with HIV. Lancet Oncol. 2021;22:e240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shive CL, Freeman ML, Younes SA, Kowal CM, Canaday DH, Rodriguez B, Lederman MM, Anthony DD. Markers of T cell exhaustion and senescence and their relationship to plasma TGF-β levels in treated HIV + Immune Non-Responders. Front Immunol. 2021;12. 10.3389/fimmu.2021.638010. [DOI] [PMC free article] [PubMed]
- 5.Imamichi H, Smith M, Adelsberger JW, Izumi T, Scrimieri F, Sherman BT, Rehm CA, Imamichi T, Pau A, Catalfamo M et al. Defective HIV-1 Proviruses Produce Viral Proteins. Proceedings of the National Academy of Sciences (PNAS). 2020, 10.1073/pnas.1917876117/-/DCSupplemental [DOI] [PMC free article] [PubMed]
- 6.World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring. Recommendations for a Public Health Approach.; 2021. ISBN 9789240031593. [PubMed]
- 7.Yan L, Xu K, Xiao Q, Tuo L, Luo T, Wang S, Yang R, Zhang F, Yang X. Cellular and molecular insights into incomplete immune recovery in HIV/AIDS patients. Front Immunol. 2023;14:1–15. 10.3389/fimmu.2023.1152951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bono V, Augello M, Tincati C, Marchetti G. Failure of CD4 + T-Cell recovery upon Virally-Effective CART: an enduring gap in the Understanding of HIV + Immunological Non-Responders. New Microbiol. 2022;45:155–72. [PubMed] [Google Scholar]
- 9.Yang X, Zhang T, Su B, Zhang X, Liu Y, Wu H. Incomplete immune reconstitution in HIV / AIDS patients on antiretroviral therapy: challenges of immunological Non-Responders. 2020, 1–16, 10.1002/JLB.4MR1019-189R [DOI] [PMC free article] [PubMed]
- 10.Veloso Carvalho-Silva WH, Andrade-Santos JL, Dos Santos Guedes MC, Guimarães RL. Genetics and immunological recovery with antiretroviral treatment for HIV. Volume 21. Pharmacogenomics; 2020. pp. 979–83. [DOI] [PubMed]
- 11.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol. 2012;2012. 10.1155/2012/670957. [DOI] [PMC free article] [PubMed]
- 12.Andrade-Santos JL, Carvalho-Silva WHV, Souto FO, Crovella S, Guimarães RL. Differences in pyroptosis of recent thymic emigrants CD4 + T lymphocytes in ART-Treated HIV-Positive patients are influenced by sex. Immunogenetics. 2021;73:349–53. 10.1007/s00251-020-01202-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Ruan L. Recent advances in poor HIV immune reconstitution: what will the future look like?? Front Microbiol 2023, 14. [DOI] [PMC free article] [PubMed]
- 14.Chen Q, Zhao Y, Zhang Y, Zhang J, Lu W, Chang CH, Jiang S. HIV associated cell death: Peptide-Induced apoptosis restricts viral transmission. Front Immunol. 2023;14. 10.3389/fimmu.2023.1096759. [DOI] [PMC free article] [PubMed]
- 15.Bertheloot D, Latz E, Franklin BS, Necroptosis. Pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park W, Wei S, Kim BS, Kim B, Bae SJ, Chae YC, Ryu D, Ha KT. Diversity and complexity of cell death: A historical review. Exp Mol Med. 2023;55:1573–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan J, Ofengeim DA. Guide to cell death pathways. Nat Rev Mol Cell Biol. 2024;25:379–95. [DOI] [PubMed] [Google Scholar]
- 18.Fu Q, Fu TM, Cruz AC, Sengupta P, Thomas SK, Wang S, Siegel RM, Wu H, Chou JJ. Structural basis and functional role of intramembrane trimerization of the Fas/CD95 death receptor. Mol Cell. 2016;61:602–13. 10.1016/j.molcel.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabea Ekabe C, Asaba Clinton N, Agyei EK, Kehbila J. Role of Apoptosis in HIV Pathogenesis. Adv Virol 2022, 2022. [DOI] [PMC free article] [PubMed]
- 20.Mehrbod P, Ande SR, Alizadeh J, Rahimizadeh S, Shariati A, Malek H, Hashemi M, Glover KKM, Sher AA, Coombs KM, et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019;10:376–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- 23.Negredo E, Massanella M, Puig J, Pérez-Álvarez N, Gallego-Escuredo JM, Villarroya J, Villarroya F, Moltó J, Santos JR, Clotet B, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-Infected patients: clinical implications. Clin Infect Dis. 2010;50:1300–8. 10.1086/651689. [DOI] [PubMed] [Google Scholar]
- 24.Guo FP, Li YJ, Qiu ZF, Lv W, Han Y, Xie J, Li YL, Song XJ, Du SS, Mehraj V, et al. Baseline Naive CD4 + T-Cell level predicting immune reconstitution in treated HIV-Infected late presenters. Chin Med J (Engl). 2016;129:2683–90. 10.4103/0366-6999.193460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dos Santos Guedes MC, Carvalho-Silva WHV, Andrade-Santos JL, Brelaz-de-Castro MCA, Souto FO, Guimarães RL. Thymic exhaustion and increased immune activation are the main mechanisms involved in impaired immunological recovery of HIV-Positive patients under ART. Viruses. 2023;15. 10.3390/v15020440. [DOI] [PMC free article] [PubMed]
- 26.Carvalho-Silva WHV, Andrade-Santos JL, Guedes MC dos, Crovella S, Guimarães S. R.L. CCR5 Genotype and Pre-Treatment CD4 + T-Cell Count Influence Immunological Recovery of HIV-Positive Patients during Antiretroviral Therapy. Gene 2020, 741, 144568. 10.1016/j.gene.2020.144568 [DOI] [PubMed]
- 27.Song A, Liu X, Huang X, Meyers K, Oh DY, Hou J, Xia W, Su B, Wang N, Lu X, et al. From CD4-Based initiation to treating all HIV-Infected adults immediately: an Evidence-Based Meta-Analysis. Front Immunol. 2018;9:1–9. 10.3389/fimmu.2018.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization Managing Advanced HIV disease and rapid initiation of antiretroviral therapy; 2017. [PubMed]
- 29.Hansjee N, Kaufmann GR, Strub C, Weber R, Battegay M, Erb P. Persistent apoptosis in HIV-1-Infected individuals receiving potent antiretroviral therapy is associated with poor recovery of CD4 T lymphocytes. J Acquir Immune Defic Syndr 2004. [DOI] [PubMed]
- 30.Molina-Pinelo S, Vallejo A, Díaz L, Soriano-Sarabia N, Ferrando-Martínez S, Resino S, Muñoz-Fernández MÁ, Leal M. Premature Immunosenescence in HIV-Infected patients on highly active antiretroviral therapy with Low-Level CD4 T cell repopulation. J Antimicrob Chemother. 2009;64:579–88. 10.1093/jac/dkp248. [DOI] [PubMed] [Google Scholar]
- 31.Kolte L. Thymic function in HIV-Infection. Dan Med J. 2013;60:44–52. [PubMed] [Google Scholar]
- 32.Massanella M, Negredo E, Pérez-Álvarez N, Puig J, Ruiz-Hernández R, Bofill M, Clotet B, Blanco J. CD4 T-Cell hyperactivation and susceptibility to cell death determine poor CD4 T-Cell recovery during suppressive HAART. AIDS. 2010;24:959–68. 10.1097/QAD.0b013e328337b957. [DOI] [PubMed] [Google Scholar]
- 33.Scherpenisse M, Kootstra NA, Bakker M, Berkhout B, Pasternak AO. Cell-Associated Hiv-1 Unspliced-to-Multiply-Spliced Rna Ratio at 12 Weeks of Art Predicts Immune Reconstitution on Therapy. mBio 2021, 12, 1–25. 10.1128/mBio.00099-21 [DOI] [PMC free article] [PubMed]
- 34.Massanella M, Gómez-Mora E, Carrillo J, Curriu M, Ouchi D, Puig J, Negredo E, Cabrera C, Clotet B, Blanco J. Increased ex vivo cell death of central memory CD4 T cells in treated HIV infected individuals with unsatisfactory immune recovery. J Transl Med. 2015;13. 10.1186/s12967-015-0601-2. [DOI] [PMC free article] [PubMed]
- 35.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Dhummakupt A, Khetan P, Nilles T, Zhou W, Mudvari P, Szewczyk J, Chen YH, Boritz E, Ji H, et al. Immune activation and exhaustion marker expression on T-Cell subsets in ART-Treated adolescents and young adults with perinatal HIV-1 infection as correlates of viral persistence. Front Immunol. 2023;14. 10.3389/fimmu.2023.1007626. [DOI] [PMC free article] [PubMed]
- 37.Zhao J, Schank M, Wang L, Li Z, Nguyen LN, Dang X, Cao D, Khanal S, Nguyen LNT, Thakuri BKC, et al. Mitochondrial functions are compromised in CD4 T cells from ART-Controlled PLHIV. Front Immunol. 2021;12. 10.3389/fimmu.2021.658420. [DOI] [PMC free article] [PubMed]
- 38.Nasi M, Pinti M, Bugarini R, Troiano L, Lugli E, Bellodi C, Mussini C, Borghi V, Trenti T, Balli F, et al. Genetic polymorphisms of Fas (CD95) and Fas ligand (CD178) influence the rise in CD4 + T cell count after antiretroviral therapy in Drug-Naïve HIV-Positive patients. Immunogenetics. 2005;57:628–35. 10.1007/s00251-005-0031-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.