Abstract
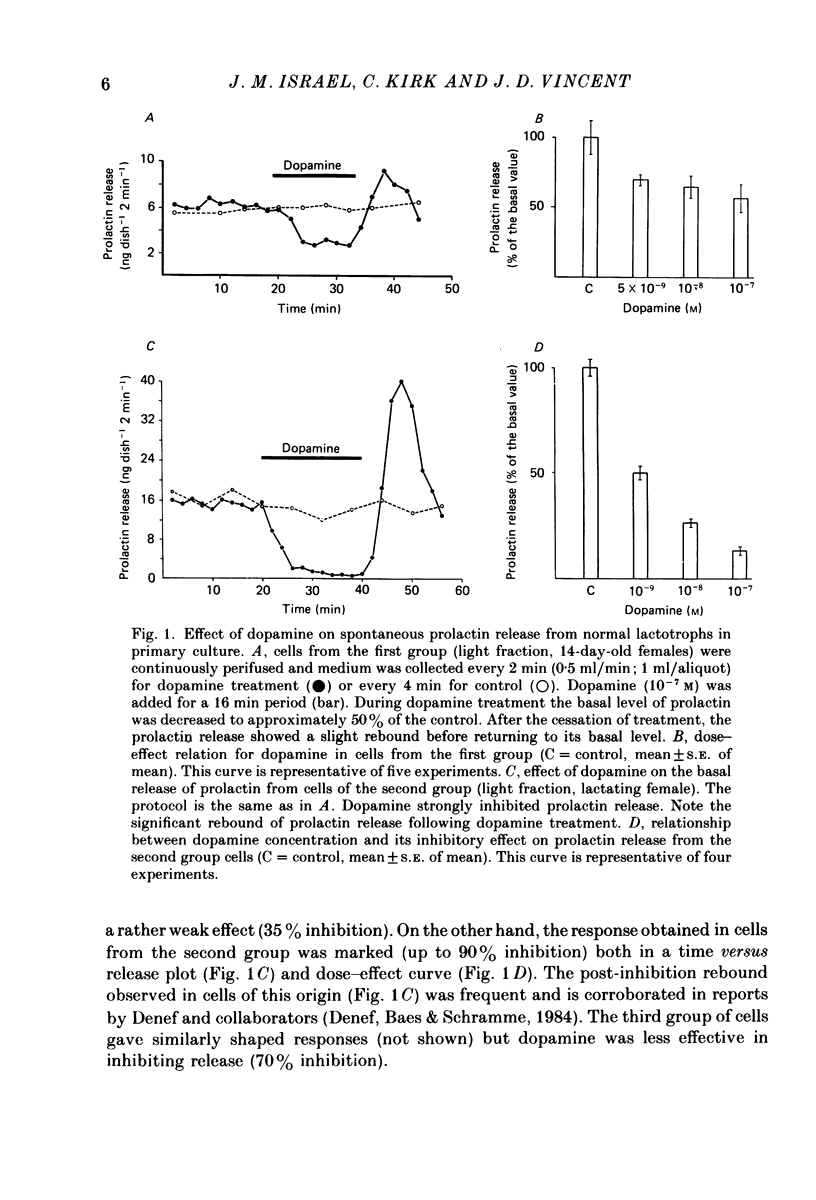
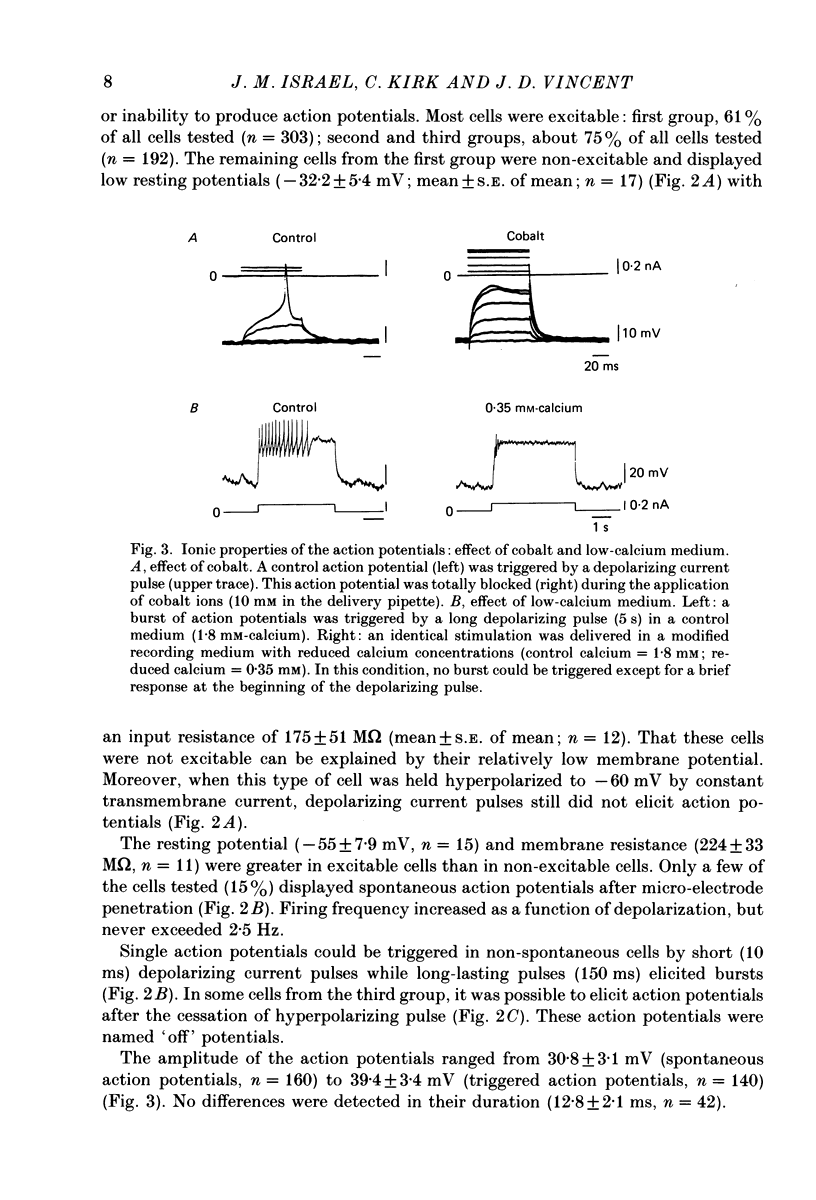
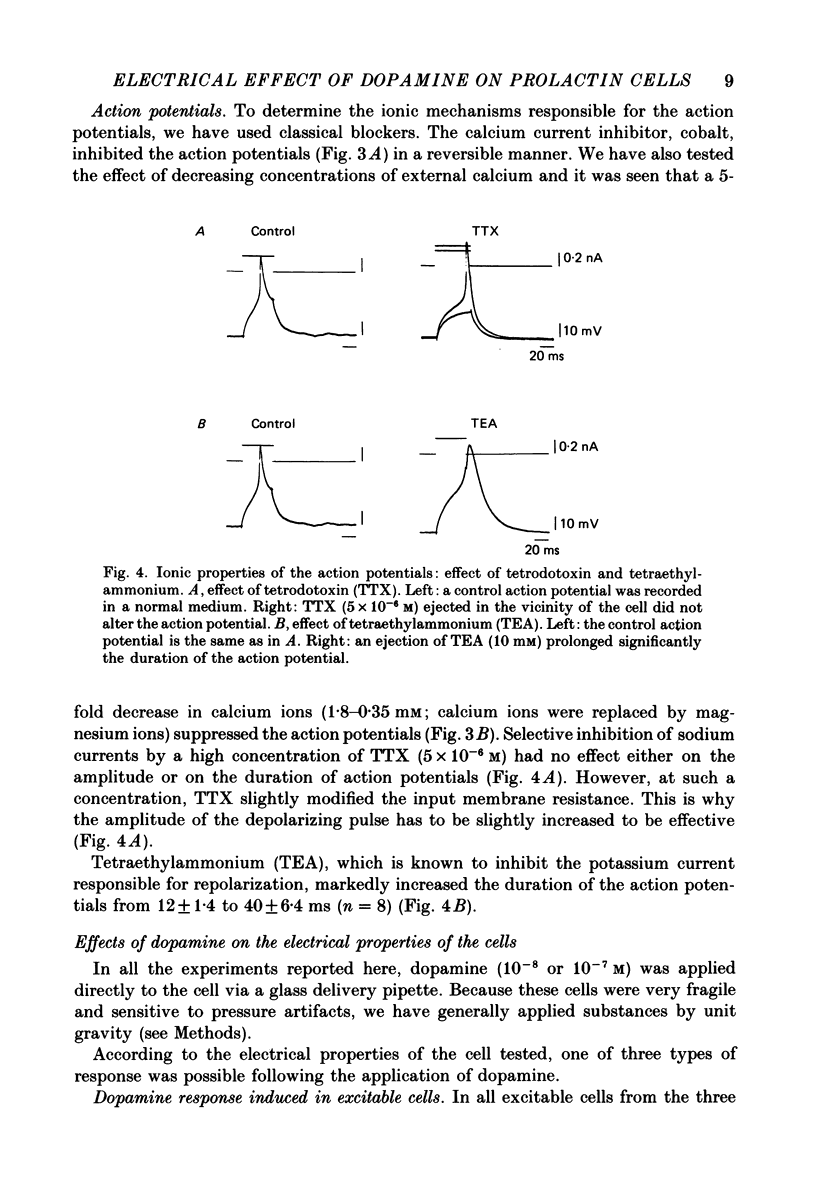
1. Cells from 14-day-old and lactating female rat pituitary glands were dissociated, separated and enriched on a continuous gradient of bovine serum albumin at unit gravity. They were maintained for at least 6 days in culture before perifusion and electrophysiological experiments were performed. 2. Immunofluorescent staining of the resulting gradient fractions (numbered F2 to F9) from both groups of animals indicated that the majority of lactotrophs were located in the light fractions (F3-F4). However, a second population of lactotrophs was observed in the heavy fractions (F7-F9) isolated from lactating females. 3. Basal secretion rates of prolactin were in the order of 2-40 ng 2 min-1 10(6) cells-1 and were inhibited by dopamine in a dose-dependent manner. 4. According to their electrophysiological properties, cells from 14-day-old females (first group) were categorized as follows: (1) inexcitable cells, which displayed a low resting potential of about -35 mV (39% of cells tested, n = 118); and (2) excitable cells, which displayed either triggered or spontaneous action potentials and resting membrane potentials higher than -50 mV (61% of cells tested, n = 185). 5. In the light fraction from lactating females (second group), the majority of the cells were excitable (70%) and showed high resting membrane potentials (-50 to -55 mV) and 15% of these cells displayed spontaneous action potentials. 6. Heavy fractions (third group) contained a high percentage of non-spontaneous but excitable cells (80% of the cells tested, n = 65). These cells were able to elicit action potentials after the cessation of hyperpolarizing current pulses ('off' potentials). 7. Action potentials were insensitive to the sodium channel blocker, tetrodotoxin (TTX; 5 x 10(-6) M) but were reversibly blocked by calcium channel blockers such as cobalt, manganese and cadmium (10 mM). 8. In excitable cells from the three groups, dopamine (10(-7) M) induced a hyperpolarizing response due to an increase of the membrane conductance. During this response, action potentials were inhibited. It was shown that this was not a direct effect of dopamine. The reversal potential of the dopamine-induced response in these cells was found to be at -100 mV. This value was shifted to more positive potentials (-50 mV) when high-potassium medium was used (56 mM). 9. In non-excitable cells (first group), dopamine (10(-7) M) induced a hyperpolarizing response due to a decrease of the membrane conductance.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
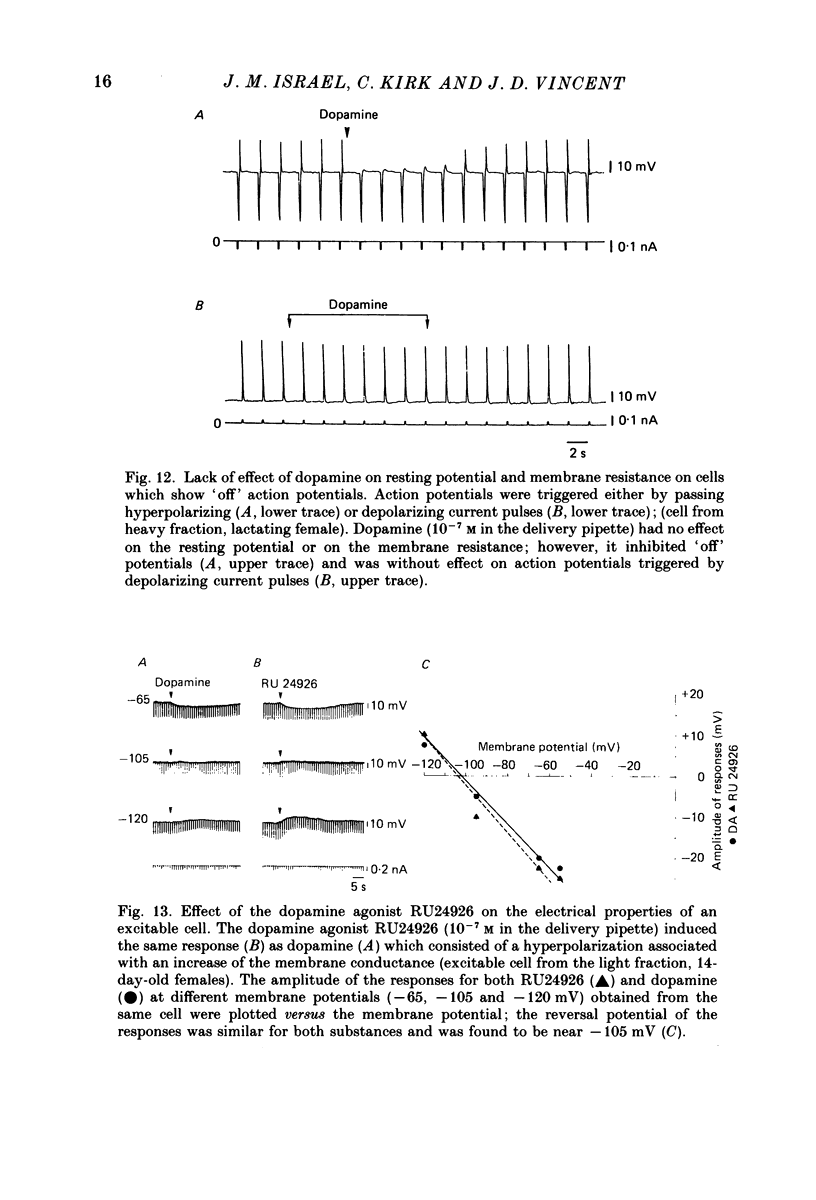
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985 Fall;6(4):564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
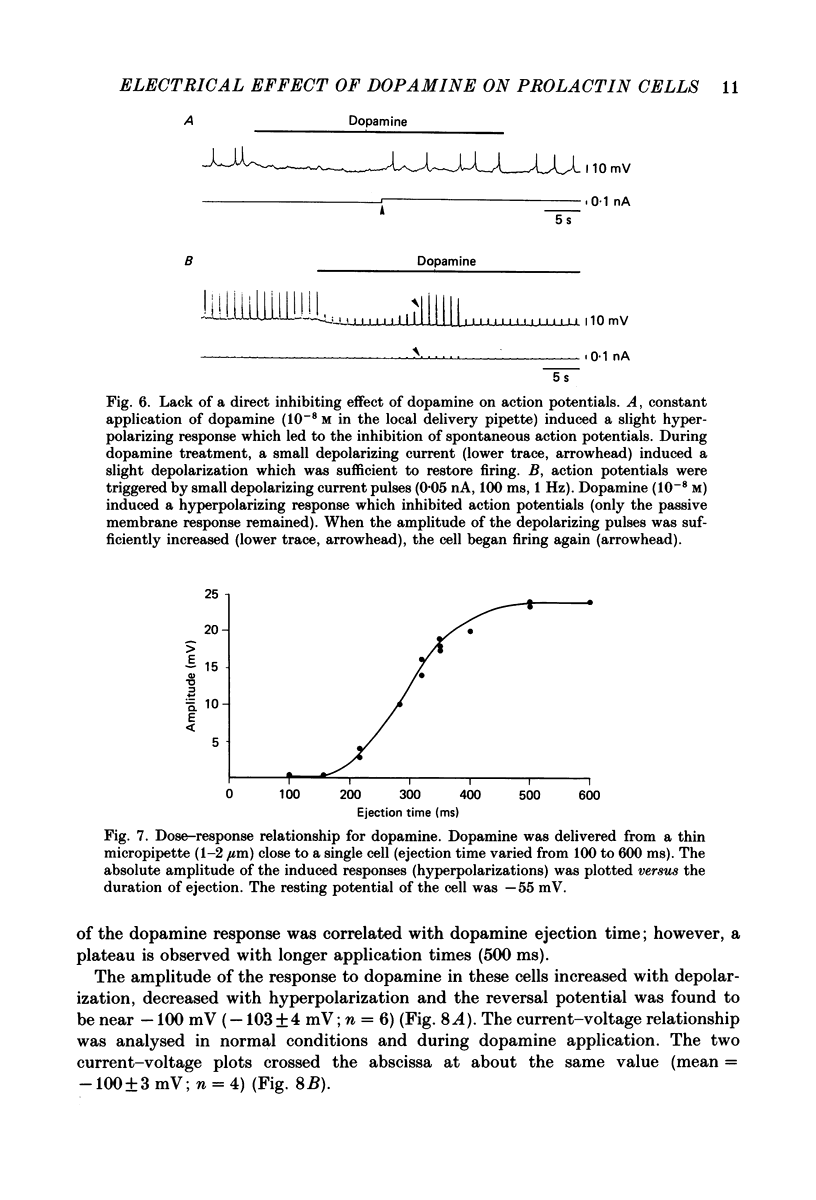
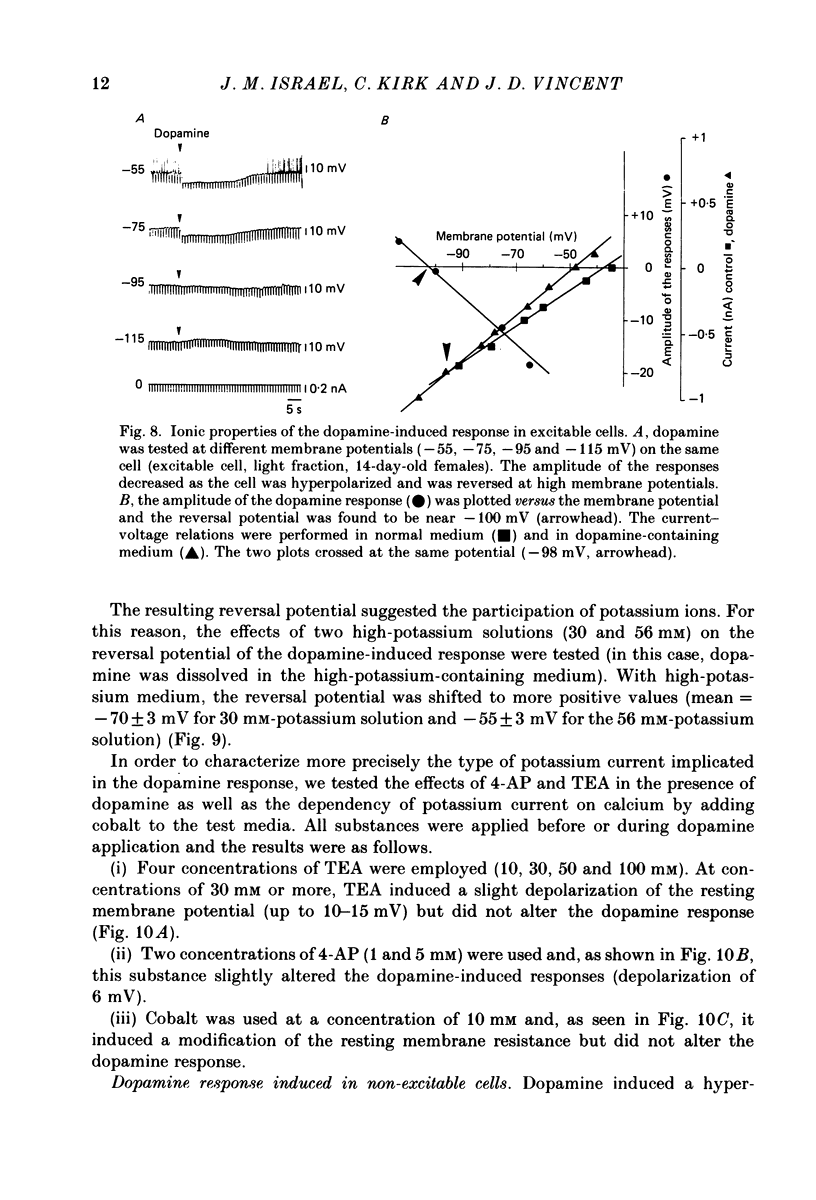
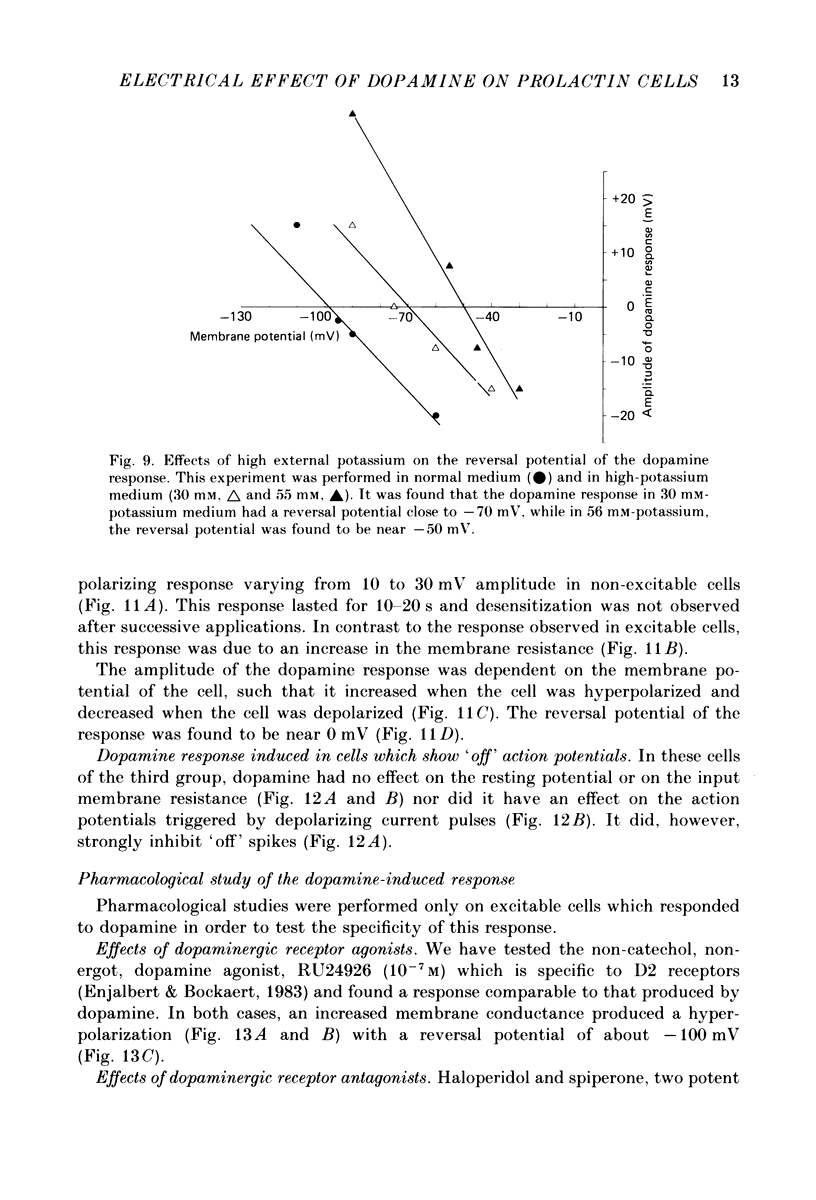
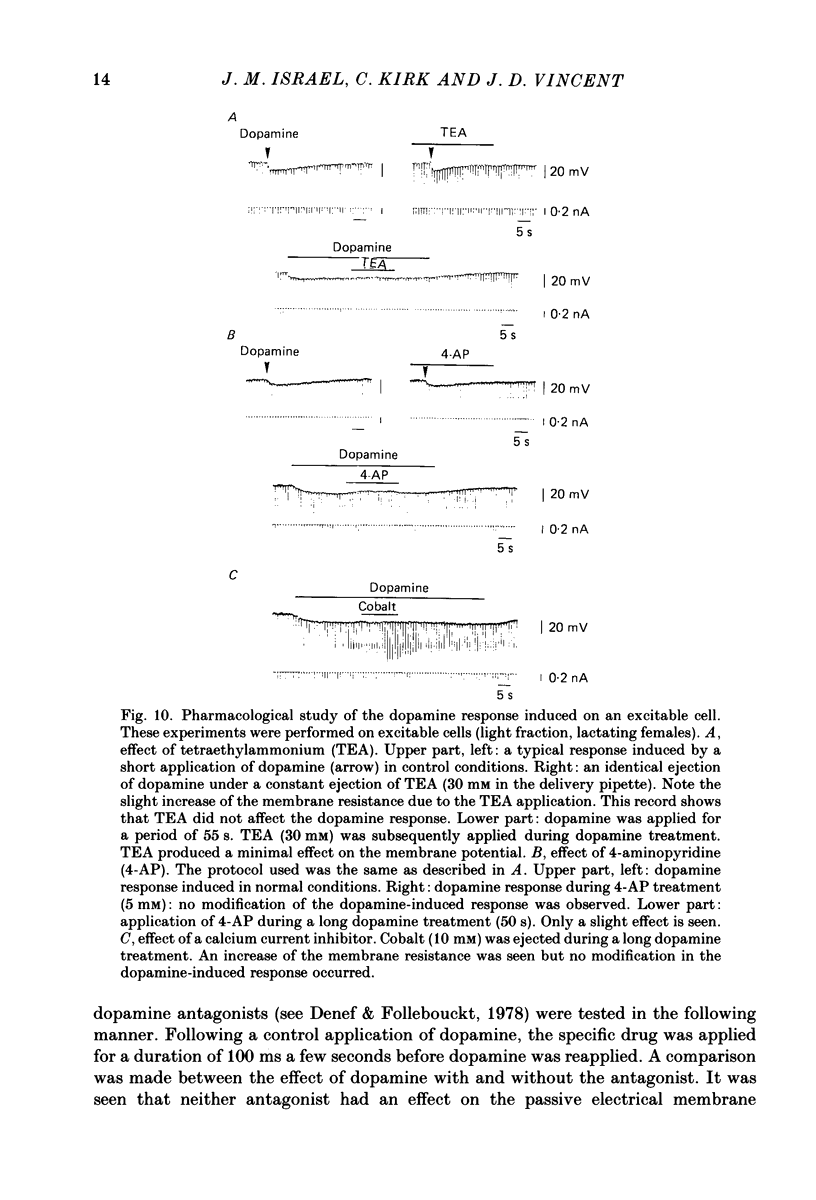
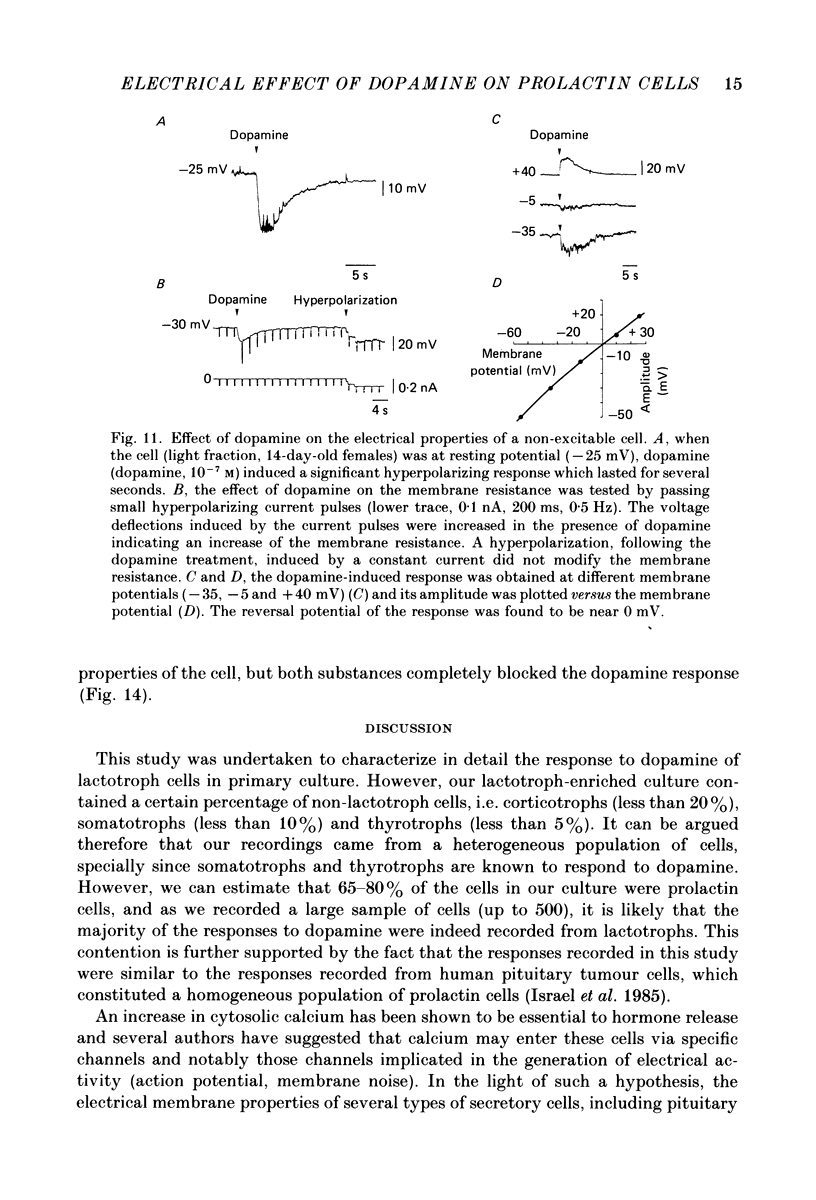
- Benardo L. S., Prince D. A. Dopamine modulates a Ca2+-activated potassium conductance in mammalian hippocampal pyramidal cells. Nature. 1982 May 6;297(5861):76–79. doi: 10.1038/297076a0. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro M. A., MacLeod R. M. Binding of dopamine to bovine anterior pituitary gland membranes. Neuroendocrinology. 1978;25(1):32–46. doi: 10.1159/000122762. [DOI] [PubMed] [Google Scholar]
- Canonico P. L., Valdenegro C. A., MacLeod R. M. The inhibition of phosphatidylinositol turnover: a possible postreceptor mechanism for the prolactin secretion-inhibiting effect of dopamine. Endocrinology. 1983 Jul;113(1):7–14. doi: 10.1210/endo-113-1-7. [DOI] [PubMed] [Google Scholar]
- Caron M. G., Beaulieu M., Raymond V., Gagné B., Drouin J., Lefkowitz R. J., Labrie F. Dopaminergic receptors in the anterior pituitary gland. Correlation of [3H]dihydroergocryptine binding with the dopaminergic control of prolactin release. J Biol Chem. 1978 Apr 10;253(7):2244–2253. [PubMed] [Google Scholar]
- Cronin M. J., Faure N., Martial J. A., Weiner R. I. Absence of high affinity dopamine receptor in GH3 cells: a prolactin-secreting clone resistant to the inhibitory action of dopamine. Endocrinology. 1980 Mar;106(3):718–723. doi: 10.1210/endo-106-3-718. [DOI] [PubMed] [Google Scholar]
- Denef C., Baes M., Schramme C. Stimulation of prolactin secretion after short term or pulsatile exposure to dopamine in superfused anterior pituitary cell aggregates. Endocrinology. 1984 Apr;114(4):1371–1378. doi: 10.1210/endo-114-4-1371. [DOI] [PubMed] [Google Scholar]
- Denef C., Follebouckt J. J. Differential effects of dopamine antagonists on prolactin secretion from cultured rat pituitary cells. Life Sci. 1978 Aug 7;23(5):431–435. doi: 10.1016/0024-3205(78)90148-0. [DOI] [PubMed] [Google Scholar]
- Denef C., Hautekeete E., De Wolf A., Vanderschueren B. Pituitary basophils from immature male and female rats: distribution of gonadotrophs and thyrotrophs as studied by unit gravity sedimentation. Endocrinology. 1978 Sep;103(3):724–735. doi: 10.1210/endo-103-3-724. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. J Physiol. 1978 Dec;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Slowing effects of dopamine and calcium-channel blockers on frequency of sodium spikes in rat pars intermedia cells. J Physiol. 1982 May;326:201–211. doi: 10.1113/jphysiol.1982.sp014186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufy B., Vincent J. D., Fleury H., Du Pasquier P., Gourdji D., Tixier-Vidal A. Dopamine inhibition of action potentials in a prolactin secreting cell line is modulated by oestrogen. Nature. 1979 Dec 20;282(5741):855–857. doi: 10.1038/282855a0. [DOI] [PubMed] [Google Scholar]
- Enjalbert A., Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol Pharmacol. 1983 May;23(3):576–584. [PubMed] [Google Scholar]
- Foord S. M., Peters J. R., Dieguez C., Scanlon M. F., Hall R. Dopamine receptors on intact anterior pituitary cells in culture: functional association with the inhibition of prolactin and thyrotropin. Endocrinology. 1983 May;112(5):1567–1577. doi: 10.1210/endo-112-5-1567. [DOI] [PubMed] [Google Scholar]
- George S. R., Watanabe M., Di Paolo T., Falardeau P., Labrie F., Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology. 1985 Aug;117(2):690–697. doi: 10.1210/endo-117-2-690. [DOI] [PubMed] [Google Scholar]
- Gibbs D. M., Neill J. D. Dopamine levels in hypophysial stalk blood in the rat are sufficient to inhibit prolactin secretion in vivo. Endocrinology. 1978 Jun;102(6):1895–1900. doi: 10.1210/endo-102-6-1895. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Farquhar M. G. Hormone secretion by cells dissociated from rat anterior pituitaries. J Cell Biol. 1973 Nov;59(2 Pt 1):276–303. doi: 10.1083/jcb.59.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel J. M., Denef C., Vincent J. D. Electrophysiological properties of normal somatotrophs in culture. An intracellular study. Neuroendocrinology. 1983 Sep;37(3):193–199. doi: 10.1159/000123542. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Jaquet P., Vincent J. D. The electrical properties of isolated human prolactin-secreting adenoma cells and their modification by dopamine. Endocrinology. 1985 Oct;117(4):1448–1455. doi: 10.1210/endo-117-4-1448. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Mechanisms and components involved in adenylate cyclase inhibition by hormones. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:135–143. [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Ingram C. D. Techniques for studying the role of electrical activity in control of secretion by normal anterior pituitary cells. Methods Enzymol. 1986;124:207–242. doi: 10.1016/0076-6879(86)24017-3. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Na and Ca channels in a transformed line of anterior pituitary cells. J Gen Physiol. 1984 Mar;83(3):371–394. doi: 10.1085/jgp.83.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J., Clemens J. A. Hypothalamic control of prolactin secretion. Vitam Horm. 1972;30:165–221. doi: 10.1016/s0083-6729(08)60796-7. [DOI] [PubMed] [Google Scholar]
- Michell B. Inositol phosphates. Profusion and confusion. Nature. 1986 Jan 16;319(6050):176–177. doi: 10.1038/319176a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Reisine T., Kebabian J. W. Adenosine 3',5'-monophosphate (cAMP)-dependent protein kinase activity in rodent pituitary tissue: possible role in cAMP-dependent hormone secretion. Endocrinology. 1984 Nov;115(5):1933–1945. doi: 10.1210/endo-115-5-1933. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Schettini G., Cronin M. J., MacLeod R. M. Adenosine 3',5'-monophosphate (cAMP) and calcium-calmodulin interrelation in the control of prolactin secretion: evidence for dopamine inhibition of cAMP accumulation and prolactin release after calcium mobilization. Endocrinology. 1983 May;112(5):1801–1807. doi: 10.1210/endo-112-5-1801. [DOI] [PubMed] [Google Scholar]
- Swennen L., Denef C. Physiological concentrations of dopamine decrease adenosine 3',5'-monophosphate levels in cultured rat anterior pituitary cells and enriched populations of lactotrophs: evidence for a causal relationship to inhibition of prolactin release. Endocrinology. 1982 Aug;111(2):398–405. doi: 10.1210/endo-111-2-398. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Catecholamines of supposed inhibitory hypophysiotrophic function suppress action potentials in prolactin cells. Nature. 1978 Dec 21;276(5690):832–834. doi: 10.1038/276832a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]



