Abstract
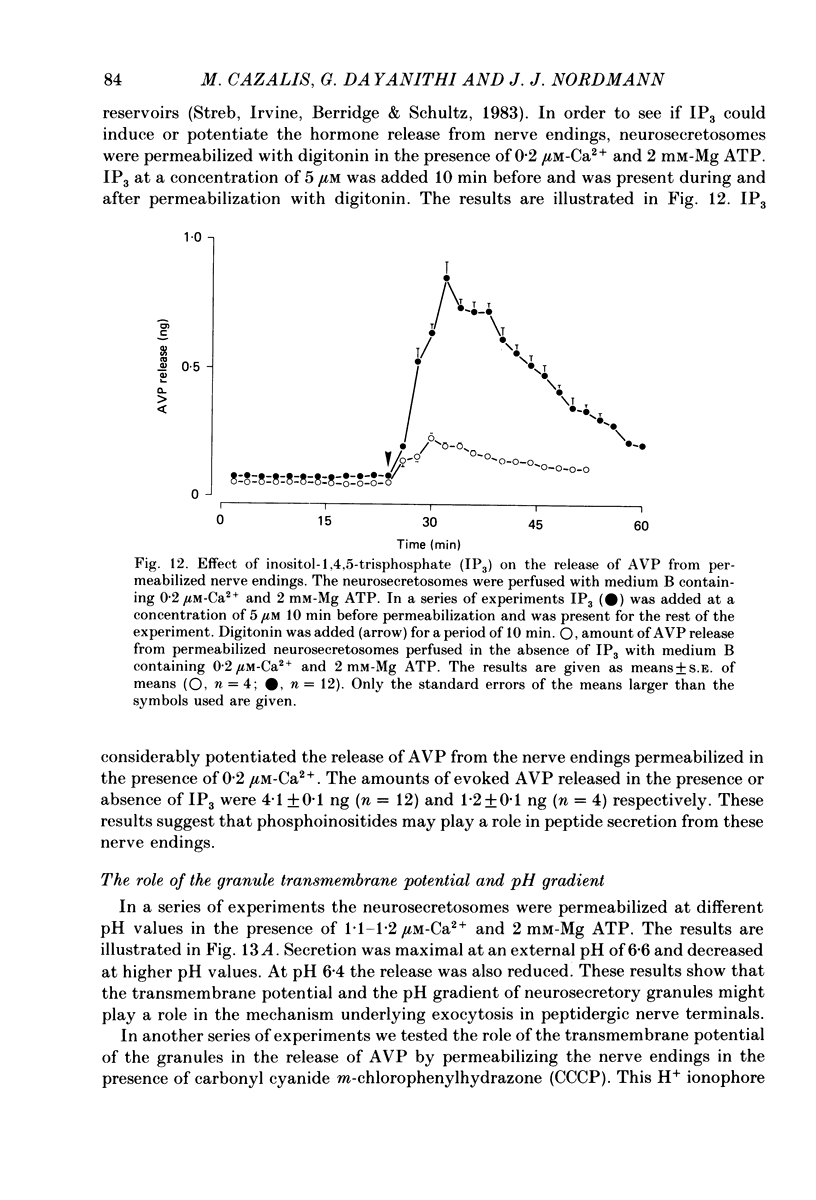
1. Isolated nerve endings from rat neurohypophyses were permeabilized with digitonin in order to gain access to the cytoplasm. Release of vasopressin (AVP), oxytocin and the neurophysins was studied under different experimental conditions. 2. Hormone release, which occurred by exocytosis, was Ca2+ dependent. Half-maximal release was observed at ca. 1.7 microM-Ca2+ in contrast to ca. 300 microM for K+-induced hormone secretion from non-permeabilized neurosecretosomes. 3. Release also occurred when the neurosecretosomes were challenged with Ca2+ 20 min after digitonin treatment. This suggests that the isolated nerve endings remain permeable after treatment with digitonin. 4. Although hormone release was potentiated in the presence of ATP, and to a lesser extent with guanosine triphosphate (GTP), secretion occurred in the absence of nucleotides. 5. Replacement of K+ as the major cation by Na+ did not modify the secretory response to a Ca2+ challenge. Release, although reduced, still occurred when KCl was replaced by sucrose. 6. Compared to glutamate, Cl-, Br- and I- did not modify the Ca2+-independent release. This release was increased in the presence of SCN-. The order of effectiveness of the anions studied in inhibiting the Ca2+-dependent release was glutamate less than Br- = Cl- = I- less than SCN-. 7. Increasing the osmolarity of the perfusate inhibited the Ca2+-dependent release of AVP and oxytocin. 8. Vincristine, which binds to microtubules, had no effect on the secretory process. 9. Ca2+ dependent AVP release was partially inhibited by the calmodulin antagonist trifluoroperazine. 10. Hormone release was potentiated by the protein kinase C activator, 4-beta-phorbol 12-myristate acetate (TPA). 11. Whereas 0.2 microM-Ca2+ induced a barely significant increase in AVP release, inositol 1,4,5-triphosphate, in the continued presence of 0.2 microM-Ca2+, produced a large secretory response. 12. 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid (SITS), an inhibitor of Cl- permeability, reduced the Ca2+-dependent AVP release. 13. Carbonyl cyanide m-chlorophenylhydrazone (CCCP), which reduces the transmembrane potential of isolated neurohypophysial granules, inhibited the Ca2+-dependent hormone secretion. 14. Maximal hormone release occurred at pH 6.6. 15. It is concluded that the permeabilized neurosecretosomes represent an excellent model for studying the minimal requirements for neurosecretion.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bhakdi S., Gratzl M. Minimal requirements for exocytosis. A study using PC 12 cells permeabilized with staphylococcal alpha-toxin. J Biol Chem. 1985 Oct 15;260(23):12730–12734. [PubMed] [Google Scholar]
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. W., Pollard H. B. Enhancement of Ca2+-induced catecholamine release by the phorbol ester TPA in digitonin-permeabilized cultured bovine adrenal chromaffin cells. FEBS Lett. 1985 Apr 8;183(1):107–110. doi: 10.1016/0014-5793(85)80964-9. [DOI] [PubMed] [Google Scholar]
- Brooks J. C., Treml S. Catecholamine secretion by chemically skinned cultured chromaffin cells. J Neurochem. 1983 Feb;40(2):468–473. doi: 10.1111/j.1471-4159.1983.tb11306.x. [DOI] [PubMed] [Google Scholar]
- Brooks J. C., Treml S. Effect of trifluoperazine and calmodulin on catecholamine secretion by saponin-skinned cultured chromaffin cells. Life Sci. 1984 Feb 13;34(7):669–674. doi: 10.1016/0024-3205(84)90231-5. [DOI] [PubMed] [Google Scholar]
- Buchs M., Dreifuss J. J., Grau J. D., Nordmann J. J. Strontium as a substitute for calcium in the process leading to neurohypophysial hormone secretion. J Physiol. 1972 Apr;222(2):168P–169P. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J., Barron J. Dissection of stages in exocytosis in the adrenal chromaffin cell with use of trifluoperazine. Proc R Soc Lond B Biol Sci. 1982 Aug 23;216(1202):111–115. doi: 10.1098/rspb.1982.0064. [DOI] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol. 1987 Sep;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985 Dec;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper D. L., Lee H. C. Inositol trisphosphate induces calcium release from nonmitochondrial stores i sea urchin egg homogenates. J Biol Chem. 1985 Nov 15;260(26):13947–13954. [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt D. A., Torp-Pedersen C., Thorn N. A. Effects of Ca2+ and calmodulin on cyclic nucleotide metabolism in neurosecretosomes isolated from ox neurohypophyses. Brain Res. 1981 Jan 5;204(1):121–128. doi: 10.1016/0006-8993(81)90656-9. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Elias P. M., Goerke J., Friend D. S., Brown B. E. Freeze-fracture identification of sterol-digitonin complexes in cell and liposome membranes. J Cell Biol. 1978 Aug;78(2):577–596. doi: 10.1083/jcb.78.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R. A., Holz R. W. Arachidonic acid release and catecholamine secretion from digitonin-treated chromaffin cells: effects of micromolar calcium, phorbol ester, and protein alkylating agents. J Neurochem. 1985 Jan;44(1):265–273. doi: 10.1111/j.1471-4159.1985.tb07140.x. [DOI] [PubMed] [Google Scholar]
- HALLER E. W., SACHS H., SPERELAKIS N., SHARE L. RELEASE OF VASOPRESSIN FROM ISOLATED GUINEA PIG POSTERIOR PITUITARIES. Am J Physiol. 1965 Jul;209:79–83. doi: 10.1152/ajplegacy.1965.209.1.79. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Cyclic nucleotides control a system which regulates Ca2+ sensitivity of platelet secretion. Nature. 1984 May 3;309(5963):66–68. doi: 10.1038/309066a0. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J. Ionic channels and hormone release from peptidergic nerve terminals. J Exp Biol. 1986 Sep;124:53–72. doi: 10.1242/jeb.124.1.53. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Chevallier J. The role of microvesicles in buffering [Ca2+]i in the neurohypophysis. Nature. 1980 Sep 4;287(5777):54–56. doi: 10.1038/287054a0. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Dreifuss J. J., Legros J. J. A correlation of release of polypeptide hormones and of immunoreactive neurophysin from isolated rat neurohypophyses. Experientia. 1971;27(11):1344–1345. doi: 10.1007/BF02136730. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J. Evidence for calcium inactivation during hormone release in the rat neurohypophysis. J Exp Biol. 1976 Dec;65(3):669–683. doi: 10.1242/jeb.65.3.669. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J. Stimulus-secretion coupling. Prog Brain Res. 1983;60:281–304. doi: 10.1016/S0079-6123(08)64397-6. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Smith J. S. The role of chemiosmotic lysis in the exocytotic release of insulin. Endocrinology. 1983 Sep;113(3):964–969. doi: 10.1210/endo-113-3-964. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T., Neighbors A. S., Pirkle J. A., Greider M. H. Use of a high voltage technique to determine the molecular requirements for exocytosis in islet cells. Diabetes. 1980 Nov;29(11):911–918. doi: 10.2337/diab.29.11.911. [DOI] [PubMed] [Google Scholar]
- Pocotte S. L., Frye R. A., Senter R. A., TerBush D. R., Lee S. A., Holz R. W. Effects of phorbol ester on catecholamine secretion and protein phosphorylation in adrenal medullary cell cultures. Proc Natl Acad Sci U S A. 1985 Feb;82(3):930–934. doi: 10.1073/pnas.82.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Scott J. H., Zinder O., Hotchkiss A. An osmotic mechanism for exocytosis from dissociated chromaffin cells. J Biol Chem. 1984 Jan 25;259(2):1114–1121. [PubMed] [Google Scholar]
- Pollard H. B., Tack-Goldman K., Pazoles C. J., Creutz C. E., Shulman N. R. Evidence for control of serotonin secretion from human platelets by hydroxyl ion transport and osmotic lysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5295–5299. doi: 10.1073/pnas.74.12.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Gatti G., Dozio N., Vicentini L. M., Meldolesi J. Ca2+-dependent and -independent release of neurotransmitters from PC12 cells: a role for protein kinase C activation? J Cell Biol. 1984 Aug;99(2):628–638. doi: 10.1083/jcb.99.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronning S. A., Heatley G. A., Martin T. F. Thyrotropin-releasing hormone mobilizes Ca2+ from endoplasmic reticulum and mitochondria of GH3 pituitary cells: characterization of cellular Ca2+ pools by a method based on digitonin permeabilization. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6294–6298. doi: 10.1073/pnas.79.20.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherman D., Nordmann J., Henry J. P. Existence of an adenosine 5'-triphosphate dependent proton translocase in bovine neurosecretory granule membrane. Biochemistry. 1982 Feb 16;21(4):687–694. doi: 10.1021/bi00533a016. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Alderton J. M. Calmodulin confers calcium sensitivity on secretory exocytosis. Nature. 1982 Jan 14;295(5845):154–155. doi: 10.1038/295154a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Torp-Pedersen C., Saermark T., Bundgaard M., Thorn N. A. ATP-dependent Ca2+ accumulation by microvesicles isolated from bovine neurohypophyses. J Neurochem. 1980 Sep;35(3):552–557. doi: 10.1111/j.1471-4159.1980.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Uttenthal L. O., Livett B. G., Hope D. B. Release of neurophysin together with vasopressin by a Ca 2 dependent mechanism. Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):379–380. doi: 10.1098/rstb.1971.0068. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]


