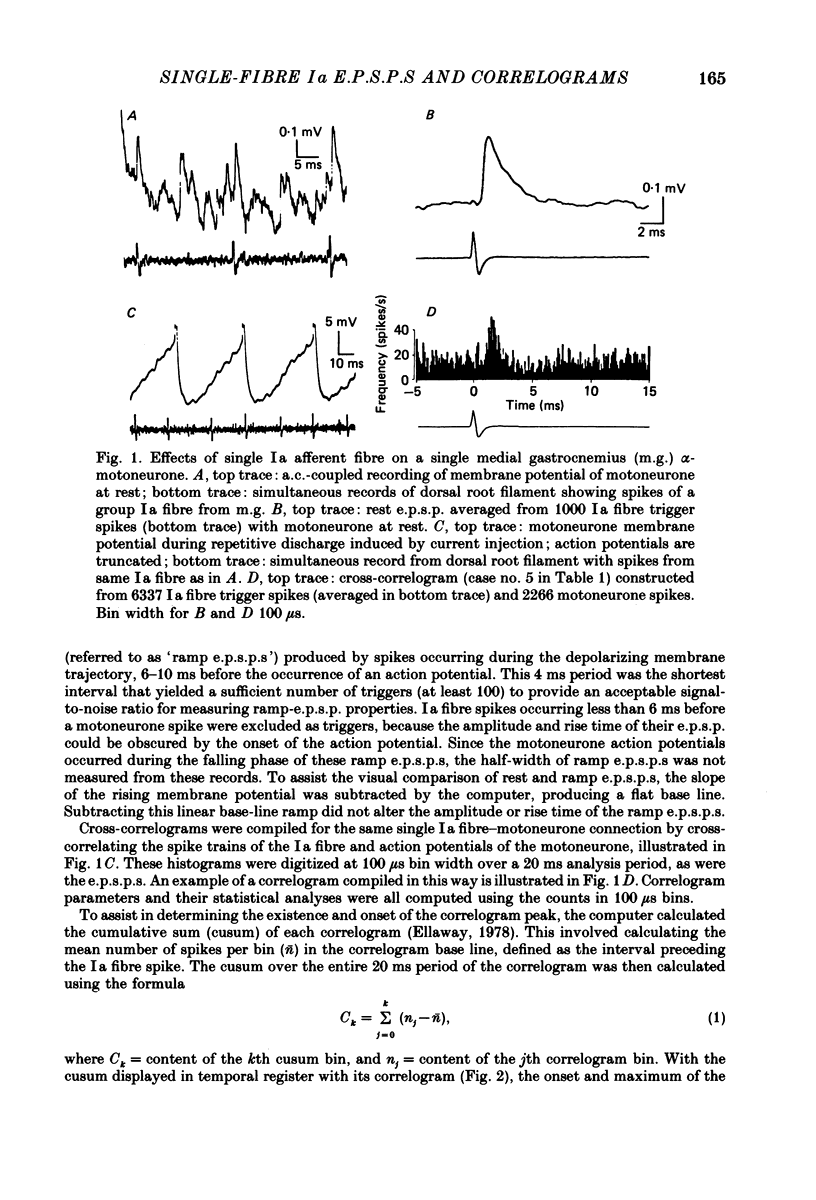
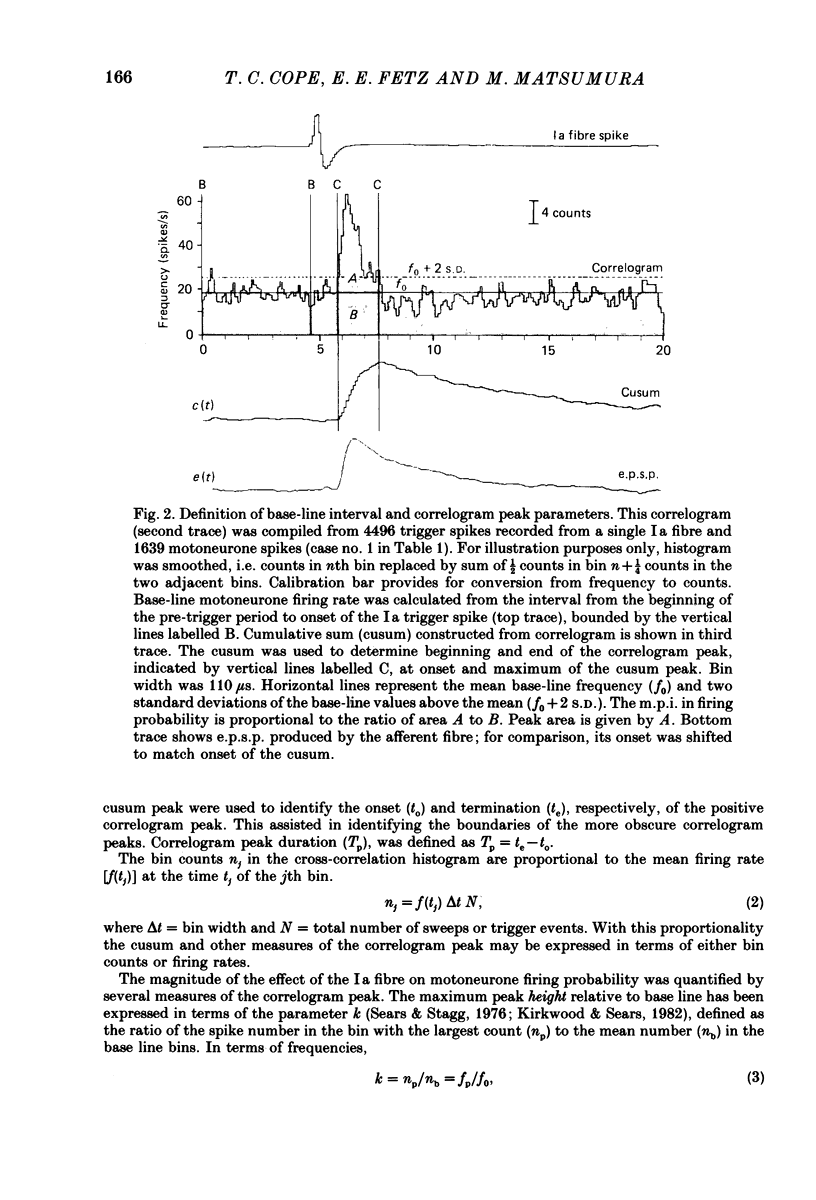
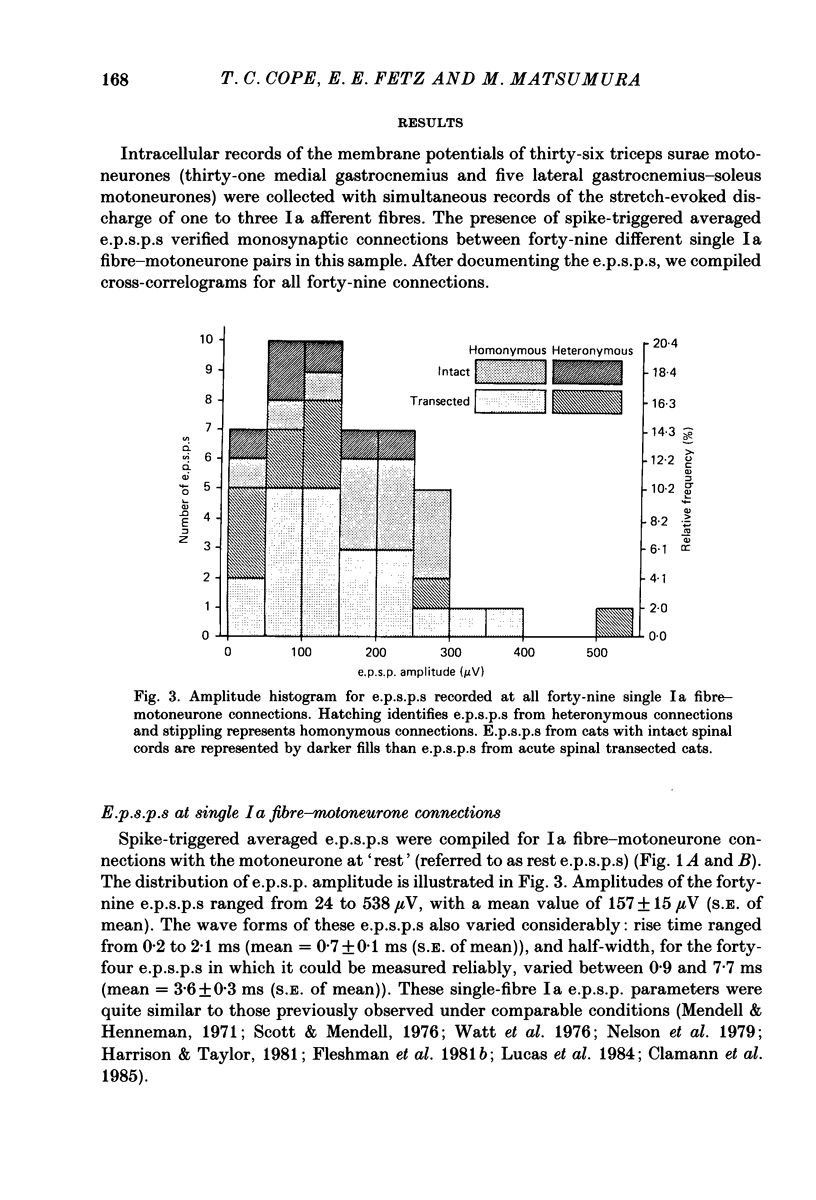
Abstract
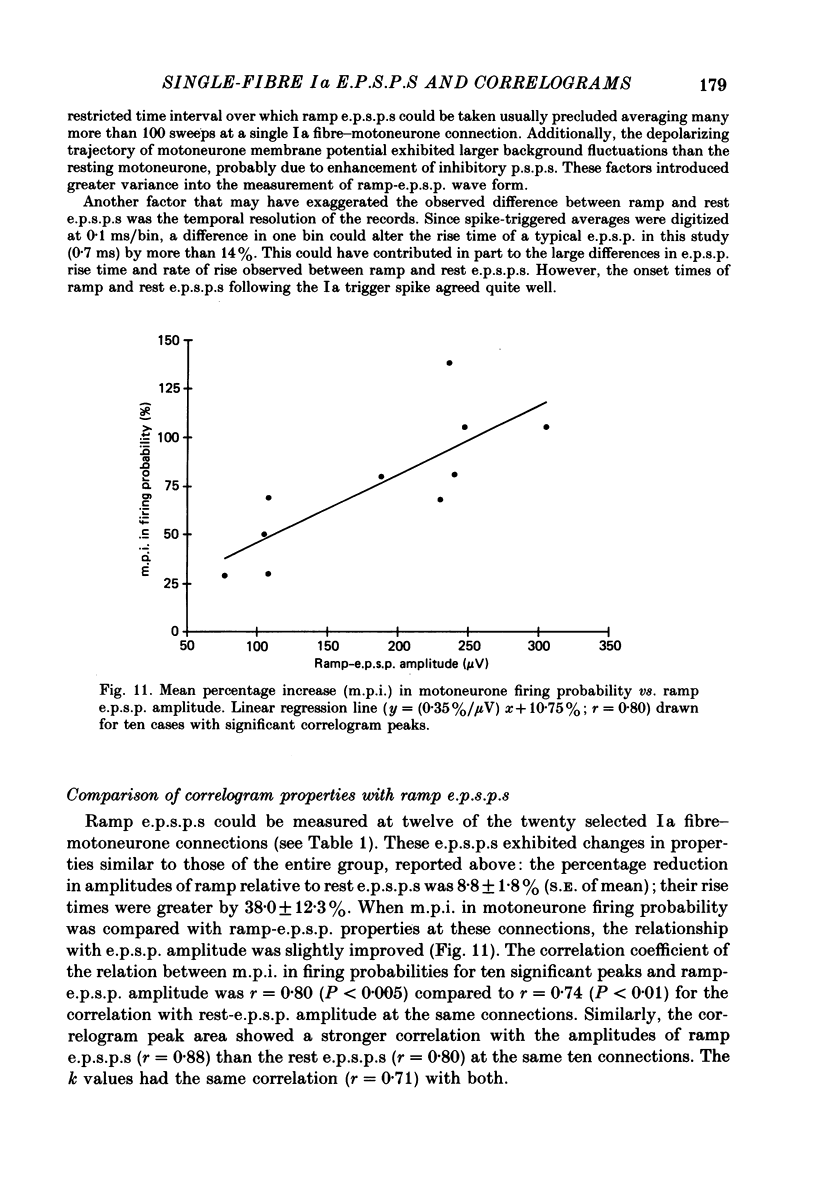
1. The relation between excitatory post-synaptic potentials (e.p.s.p.s) by single I a fibres and the resultant cross-correlograms in triceps surae motoneurones was investigated in barbiturate-anaesthetized cats. The e.p.s.p.s. were documented first, using the discharge of single Ia fibres evoked by muscle stretch to compile spike-triggered averages of motoneurone membrane potential. Subsequently, Ia fibre action potentials were cross-correlated with rhythmic discharge of the same motoneurones induced by intracellular injection of current. 2. Primary correlogram peaks were statistically significant for thirty-one of forty-nine single Ia fibre-motoneurone connections. Cumulative sums of correlograms were used to identify the onset and duration of peaks. For twenty cases involving more than 2000 trigger spikes, thirteen showed significant correlogram peaks. For these thirteen, the mean percentage increase (m.p.i.) in motoneurone firing probability, defined as the mean height of the correlogram peak above base line, ranged from 29 to 138%. The k values (maximum height divided by base line) ranged from 2.1 to 5.2. Peak duration varied from 1.8 to 3.2 ms. In the remaining seven cases the Ia e.p.s.p.s produced no significant correlogram peak (i.e. P greater than 0.05). 3. A significant positive relationship (r = 0.76; P less than 0.005) was found between m.p.i. in motoneurone firing probability and e.p.s.p. amplitude (n = 13), with a mean slope of 0.30%/microV. The k values were more weakly related to e.p.s.p. amplitude (r = 0.67; P less than 0.01). The correlogram parameter most strongly related to e.p.s.p. amplitude (r = 0.80) was correlogram peak area (number of spikes above base line per excitatory post-synaptic potential). E.p.s.p. rate of rise was not significantly related (P greater than 0.10) to either m.p.i. in firing probability (r = 0.28) or peak area (r = 0.36). 4. The shapes of the primary correlogram peaks could be accounted for largely by a function proportional to the e.p.s.p. derivative (after temporal alignment). Subtracting a function proportional to the e.p.s.p. derivative from the correlogram peak left either a negligible remainder or a remainder term whose duration was shorter than the e.p.s.p. 5. To investigate properties of single-fibre Ia e.p.s.p.s occurring near motoneurone threshold during repetitive firing, e.p.s.p.s were selectively averaged using Ia spikes occurring near the end of the depolarizing ramp in membrane potential. These 'ramp e.p.s.p.s' tended to be somewhat smaller (by ca.8%) than the 'rest e.p.s.p.s' produced at the same connections with the motoneurone at rest.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
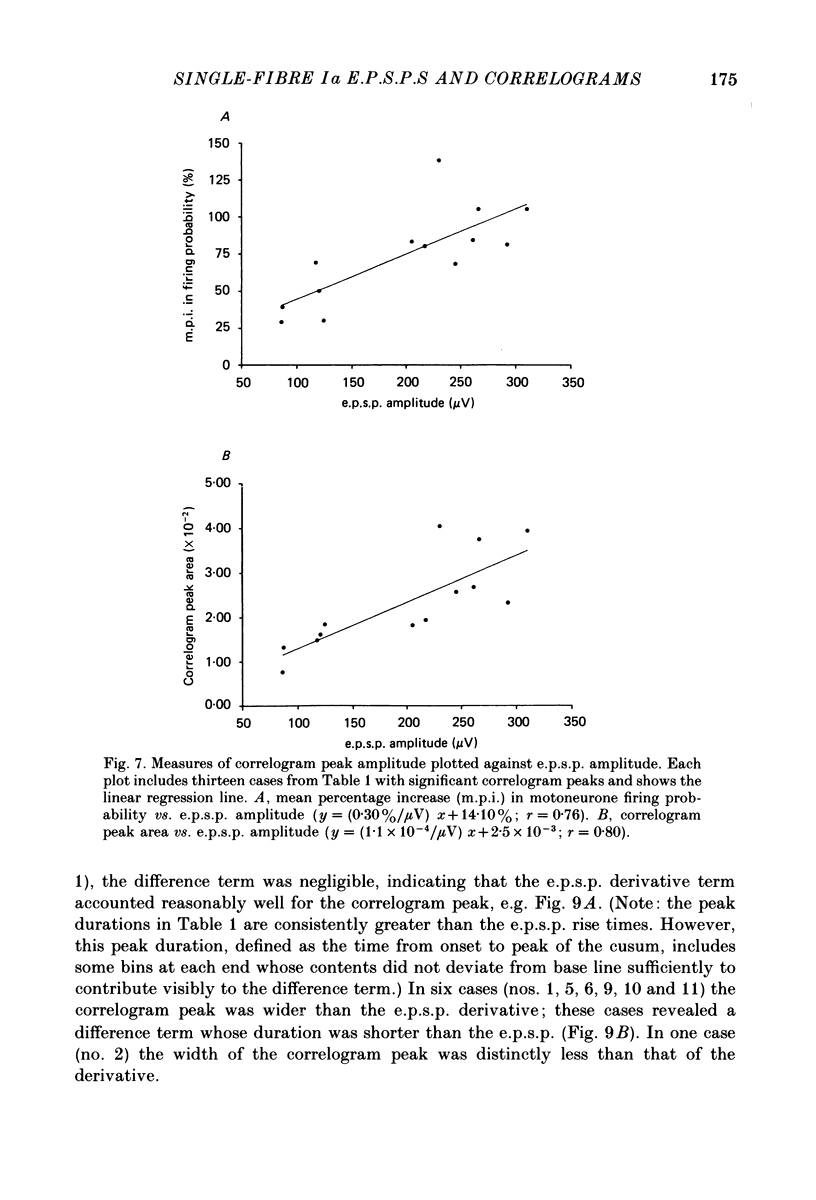
PDF





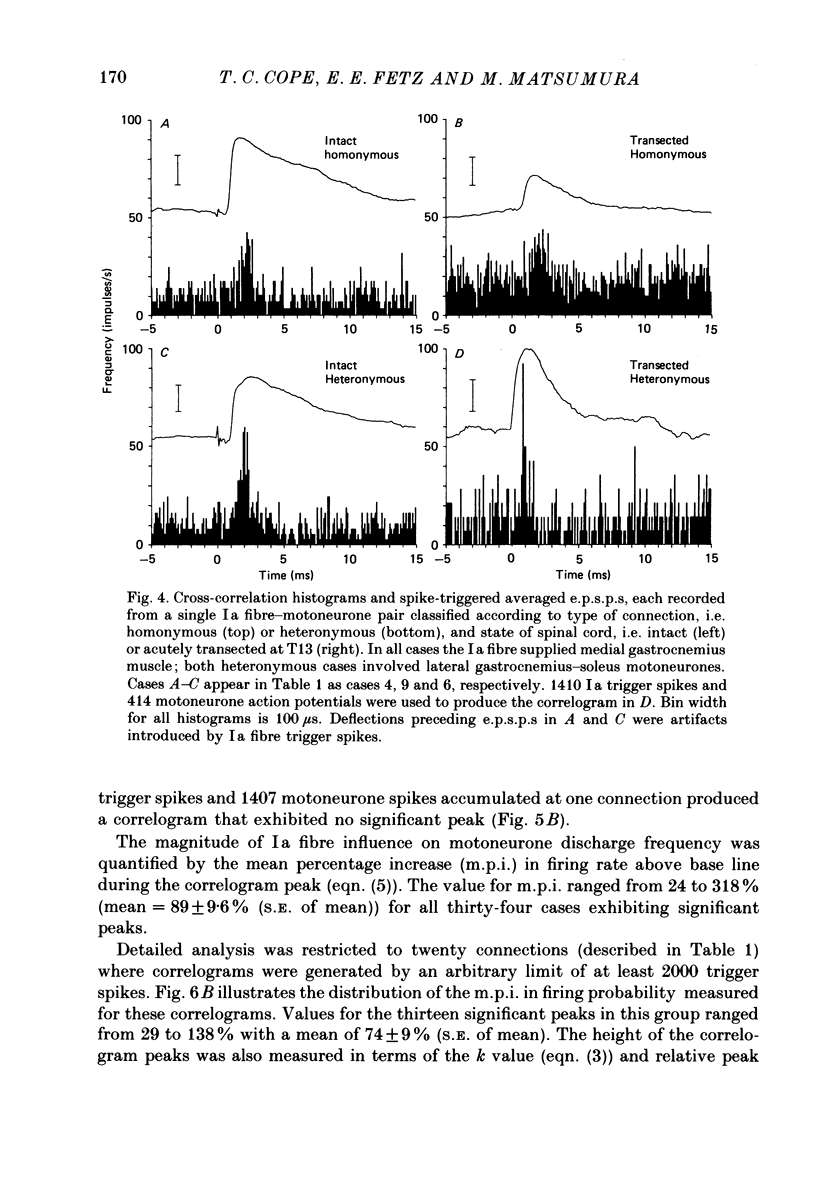
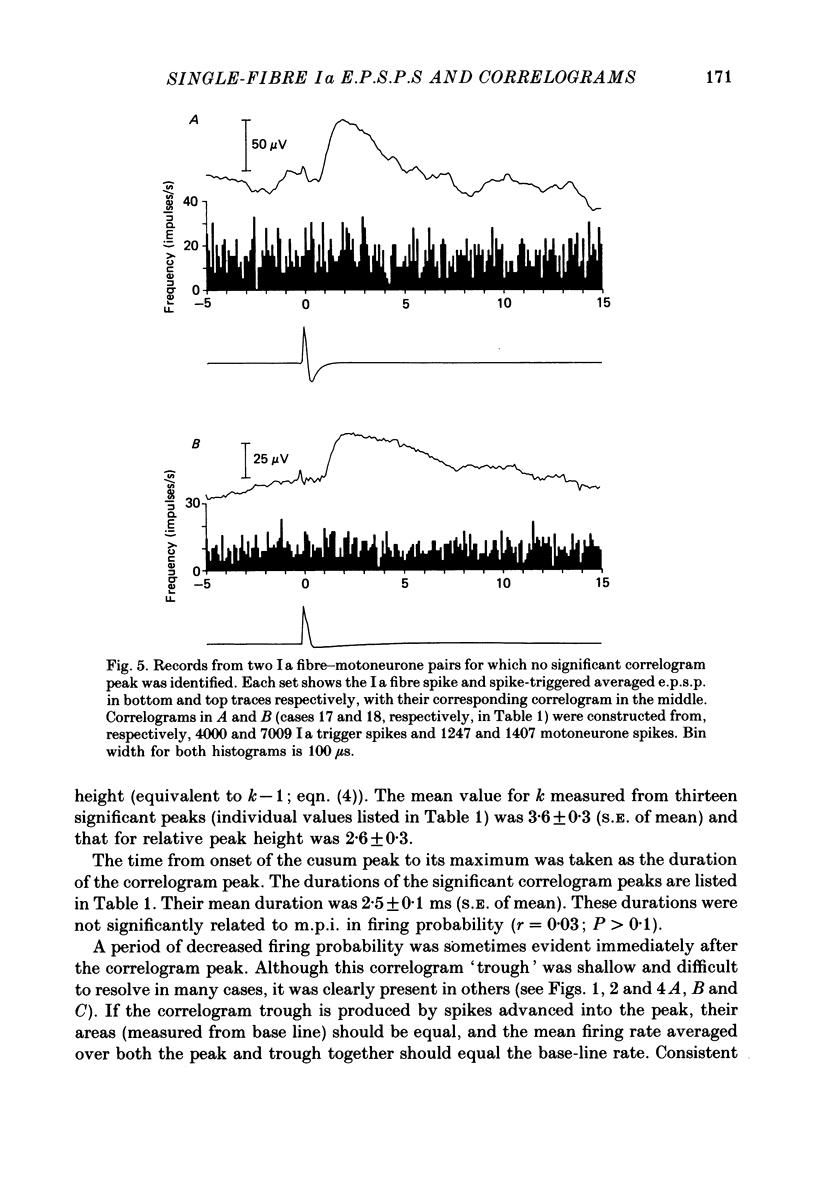
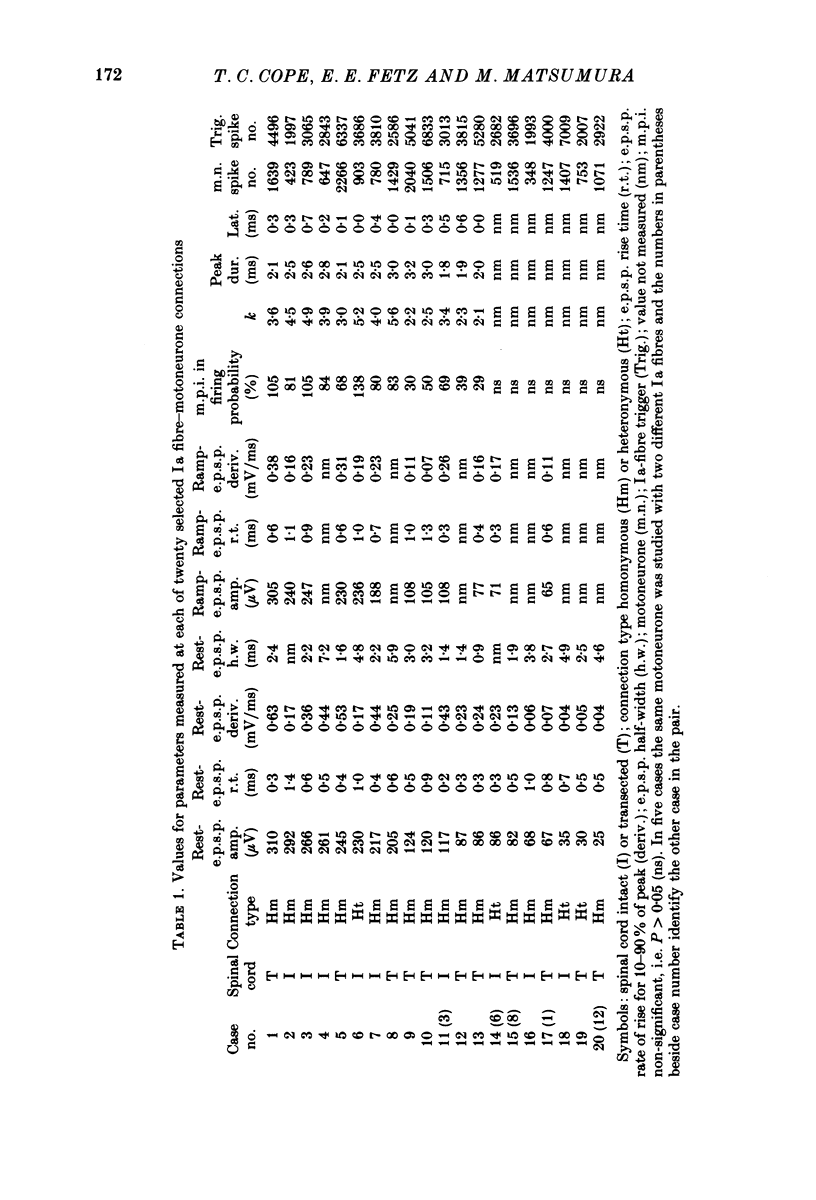
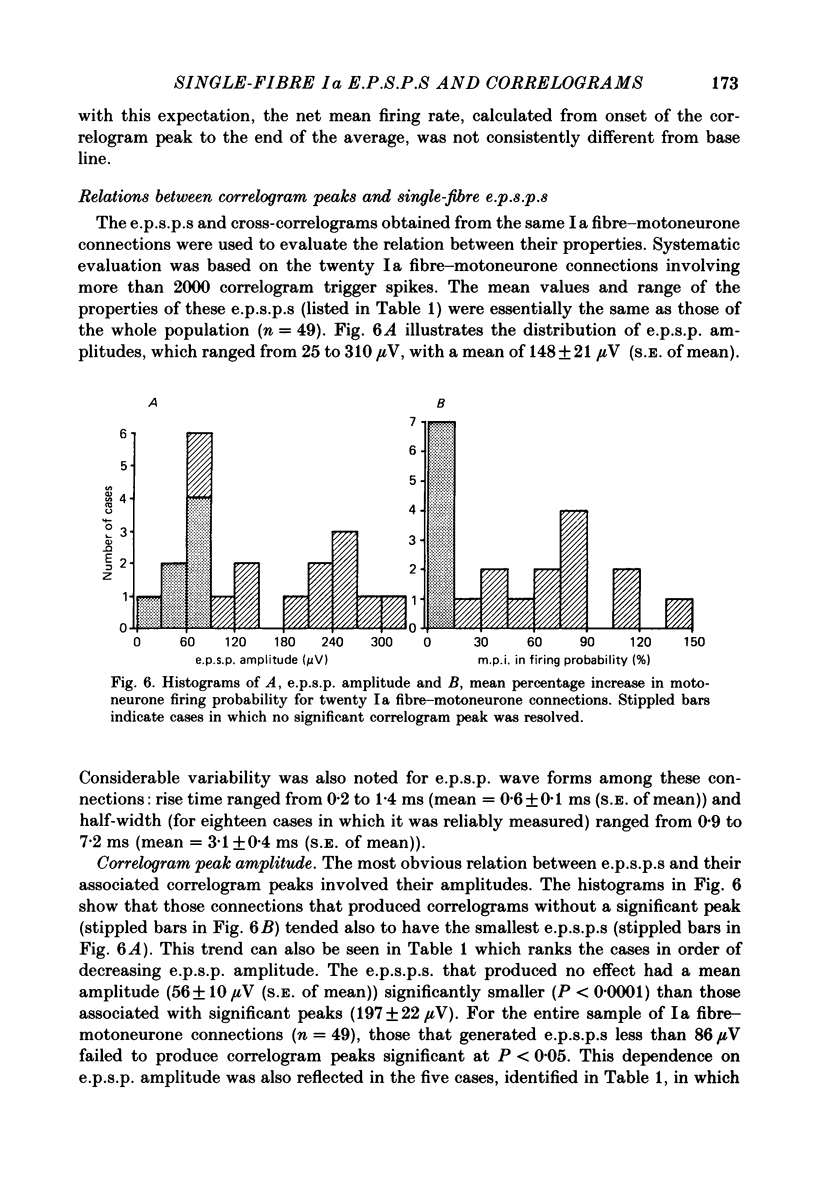

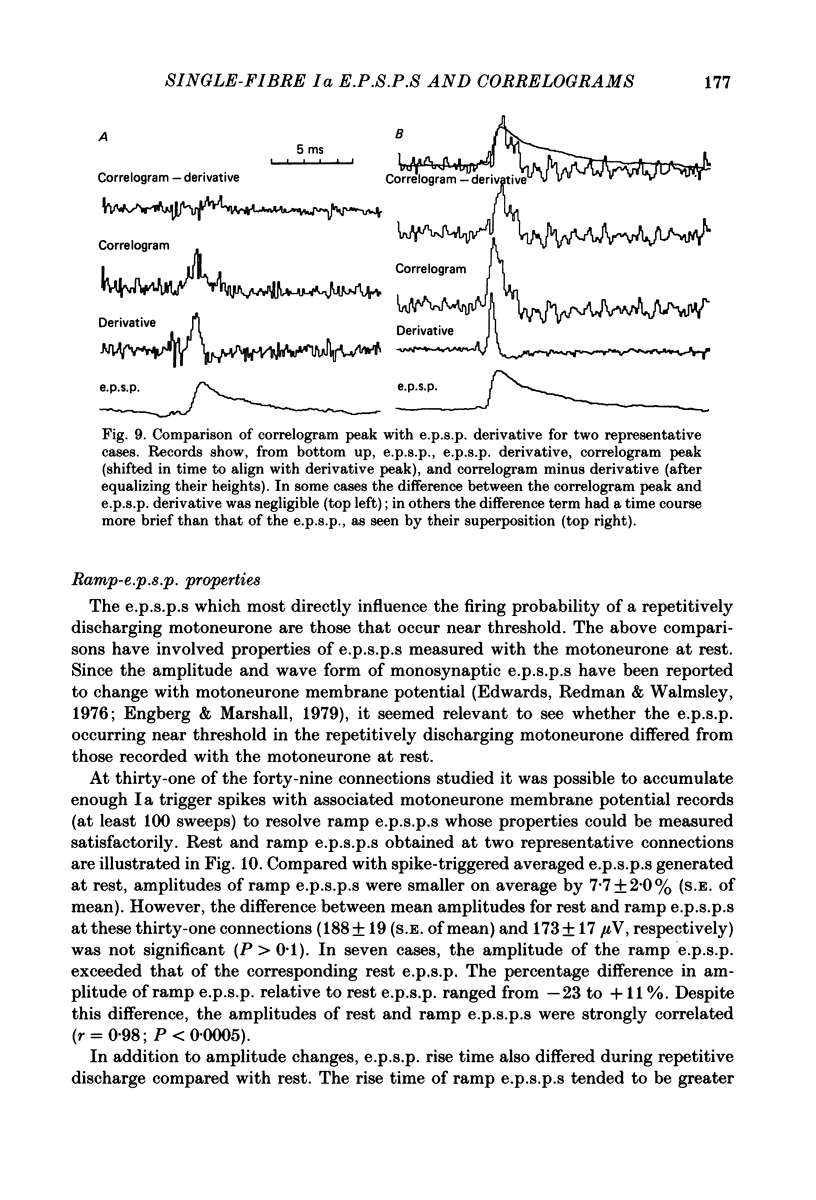









Images in this article
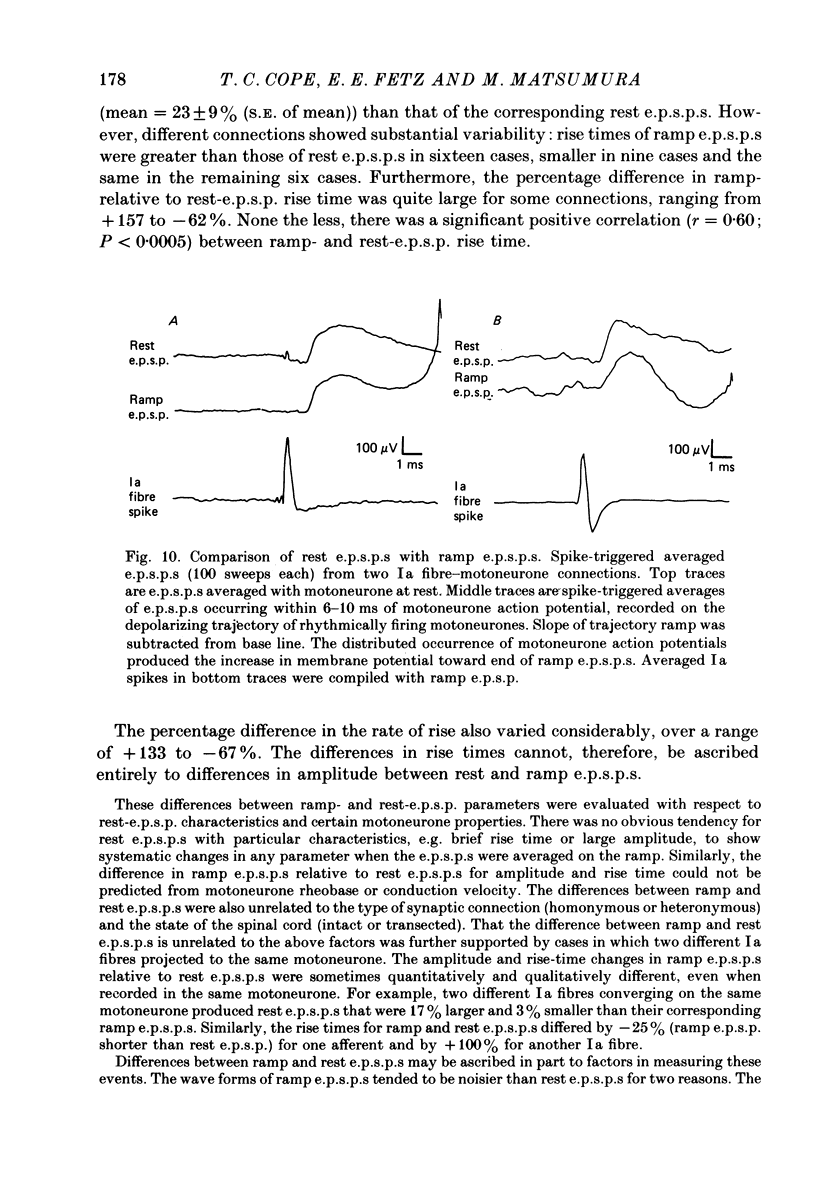
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby P., Zilm D. Relationship between EPSP shape and cross-correlation profile explored by computer simulation for studies on human motoneurons. Exp Brain Res. 1982;47(1):33–40. doi: 10.1007/BF00235883. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Binder M. D., Botterman B. R., Reinking R. M., Stuart D. G. Motor unit--muscle spindle interactions in active muscles of decerebrate cats. Neurosci Lett. 1980 Aug;19(1):55–60. doi: 10.1016/0304-3940(80)90255-4. [DOI] [PubMed] [Google Scholar]
- Clamann H. P., Henneman E., Lüscher H. R., Mathis J. Structural and topographical influences on functional connectivity in spinal monosynaptic reflex arcs in the cat. J Physiol. 1985 Jan;358:483–507. doi: 10.1113/jphysiol.1985.sp015563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W. F., 3rd, Honig M. G., Mendell L. M. Heterogeneity of group Ia synapses on homonymous alpha-motoneurons as revealed by high-frequency stimulation of Ia afferent fibers. J Neurophysiol. 1984 Nov;52(5):980–993. doi: 10.1152/jn.1984.52.5.980. [DOI] [PubMed] [Google Scholar]
- Cope T. C., Nelson S. G., Mendell L. M. Selectivity in synaptic changes caudal to acute spinal cord transection. Neurosci Lett. 1980 Dec;20(3):289–294. doi: 10.1016/0304-3940(80)90162-7. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. The effect of polarizing currents on unitary Ia excitatory post-synaptic potentials evoked in spinal motoneurones. J Physiol. 1976 Aug;259(3):705–723. doi: 10.1113/jphysiol.1976.sp011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Engberg I., Marshall K. C. Reversal potential for Ia excitatory post synaptic potentials in spinal motoneurones of cats. Neuroscience. 1979;4(11):1583–1591. doi: 10.1016/0306-4522(79)90021-6. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W., Friedman W. A. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1326–1338. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W. Homonymous projection of individual group Ia-fibers to physiologically characterized medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1339–1348. doi: 10.1152/jn.1981.46.6.1339. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol. 1980 Jun;303:351–364. doi: 10.1113/jphysiol.1980.sp013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Katz R., Malmsten J. Effects of chronic partial deafferentiation on the electrical properties of lumbar alpha-motoneurones in the cat. Brain Res. 1982 Aug 19;246(1):23–33. doi: 10.1016/0006-8993(82)90138-x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Taylor A. Individual excitatory post-synaptic potentials due to muscle spindle Ia afferents in cat triceps surae motoneurones. J Physiol. 1981 Mar;312:455–470. doi: 10.1113/jphysiol.1981.sp013638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S., Musha T., Nakajima Y., Okamoto Y. Estimation of the rising phase of EPSP analyzed by computer simulation of the coding process. Neurosci Res. 1984 Feb;1(1):53–65. doi: 10.1016/0168-0102(84)90030-0. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982 Jan;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K. Cross-correlation functions for a neuronal model. Biophys J. 1974 Aug;14(8):567–582. doi: 10.1016/S0006-3495(74)85936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K., Poppele R. E. Correlation analysis of stimulus-evoked changes in excitability of spontaneously firing neurons. J Neurophysiol. 1977 May;40(3):616–625. doi: 10.1152/jn.1977.40.3.616. [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Gerstein G. L. Interactions among an ensemble of chordotonal organ receptors and motor neurons of the crayfish claw. J Neurophysiol. 1979 Mar;42(2):383–399. doi: 10.1152/jn.1979.42.2.383. [DOI] [PubMed] [Google Scholar]
- Lucas S. M., Cope T. C., Binder M. D. Analysis of individual Ia-afferent EPSPs in a homonymous motoneuron pool with respect to muscle topography. J Neurophysiol. 1984 Jan;51(1):64–74. doi: 10.1152/jn.1984.51.1.64. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970 Sep;10(9):876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. G., Collatos T. C., Niechaj A., Mendell L. M. Immediate increase in Ia-motoneuron synaptic transmission caudal to spinal cord transection. J Neurophysiol. 1979 May;42(3):655–664. doi: 10.1152/jn.1979.42.3.655. [DOI] [PubMed] [Google Scholar]
- Nelson S. G., Mendell L. M. Projection of single knee flexor Ia fibers to homonymous and heteronymous motoneurons. J Neurophysiol. 1978 May;41(3):778–787. doi: 10.1152/jn.1978.41.3.778. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Mendell L. M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976 Jul;39(4):679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Weinberg R. J. The relationship between cross-correlation measures and underlying synaptic events. Brain Res. 1985 Apr 1;331(1):180–184. doi: 10.1016/0006-8993(85)90732-2. [DOI] [PubMed] [Google Scholar]
- Sypert G. W., Munson J. B. Basis of segmental motor control: motoneuron size or motor unit type? Neurosurgery. 1981 May;8(5):608–621. doi: 10.1227/00006123-198105000-00020. [DOI] [PubMed] [Google Scholar]
- Ulfhake B., Kellerth J. O. Electrophysiological and morphological measurements in cat gastrocnemius and soleus alpha-motoneurones. Brain Res. 1984 Jul 30;307(1-2):167–179. doi: 10.1016/0006-8993(84)90471-2. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]